��Ŀ����
��2007?�������ڵ������������л����һ�����ĺ�ͭ���ϣ��ݱ�������ͨ����������ȵ������£�ͭ��ϡ���ᷴӦת��Ϊ����ͭ����1������ɸ÷�Ӧ�Ļ�ѧ����ʽ2Cu+2H2SO4+O2
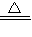 2CuSO4+______
2CuSO4+______��2����ѡ�õ�ϡ�������ʵ���������Ϊ12%������100 mL98%��Ũ���ᣨ�ܶ�Ϊ1.8g/cm3�����Ƹ�ϡ���ᣬ���ˮ______g��
��3��ȡһ��������ϡ�ͺ�����ᣨ���к�����98g����ͭǡ����ȫ��Ӧ���ٶ�������������ʷ�Ӧ����Ӧ����������ͭ��Һ��������
���𰸡��������������غ㶨����ȷ������ѧ��ʽ�е�δ֪��ѧʽ����ϡ����Һʱ����ˮʱ�����������ֲ��䣬�����ڼ���ʱ�����ʵ���������������ϵ�����������������������μӷ�Ӧ��ͭ�����������ɵ�����ͭ���������Ӷ��������ͭ��������
����⣺��1���������غ㶨�ɿ�ȷ�������������ͭ�⣬��һ��Ϊˮ��
��2�������ˮ������ΪX
100 mL×98%×1.8g/cm3=��100 mL×1.8g/cm3+X��×12%
X=1290g
��3���⣺��μӷ�Ӧ��ͭ�������������ֱ�Ϊx��y����������ͭ������Ϊz
2Cu+2H2SO4+O2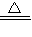 2CuSO4+2H2O
2CuSO4+2H2O
2×64 2×98 32 2×160
x 98 y z
 =
= =
= =
=
x=64��y=16��z=160
��������ͭ��Һ������Ϊ��
64+16+ =896.7g��
=896.7g��
�𣺷�Ӧ��������Һ������Ϊ896.7g��
��������ȷ����Ӧ��������Һ������ʱ�����ǿ������÷�Ӧǰ�����вμӷ�Ӧ�����ʵ���������ȥ����������Ͳ��������ʵõ���
����⣺��1���������غ㶨�ɿ�ȷ�������������ͭ�⣬��һ��Ϊˮ��
��2�������ˮ������ΪX
100 mL×98%×1.8g/cm3=��100 mL×1.8g/cm3+X��×12%
X=1290g
��3���⣺��μӷ�Ӧ��ͭ�������������ֱ�Ϊx��y����������ͭ������Ϊz
2Cu+2H2SO4+O2
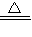 2CuSO4+2H2O
2CuSO4+2H2O2×64 2×98 32 2×160
x 98 y z
 =
= =
= =
=
x=64��y=16��z=160
��������ͭ��Һ������Ϊ��
64+16+
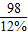 =896.7g��
=896.7g���𣺷�Ӧ��������Һ������Ϊ896.7g��
��������ȷ����Ӧ��������Һ������ʱ�����ǿ������÷�Ӧǰ�����вμӷ�Ӧ�����ʵ���������ȥ����������Ͳ��������ʵõ���

��ϰ��ϵ�д�
�����Ŀ