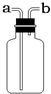
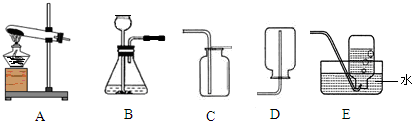��Ŀ����
���������ʵ��װ��ͼ�ش����⣺
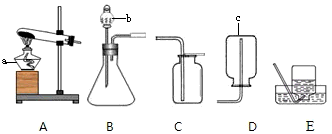
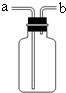
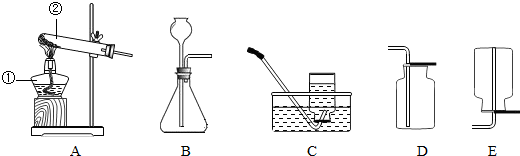
��1������a��c�����Ʒֱ���________��________��
��2������Bװ������������Ӧ�Ļ�ѧ����ʽ��________����Ӧ����Ϊ________��ʵ������ȡ������̼�ķ���װ��Ӧѡ��________����װ�ñ�ţ�����Ӧ�Ļ�ѧ����ʽ��________��
��3���������ܶ�С�ڿ�����������ˮ�����壬ʵ�����ü�����ˮ�����ƺͼ�ʯ�ҵĹ���������ȡ���飮��ȡ����ķ���װ��Ӧѡ��________����װ�ñ�ţ���ͬ�����ռ�װ��Ӧѡ________����д��������ȫȼ�յĻ�ѧ����ʽ________��
�⣺
��1��aΪ��Դ�������оƾ��ƣ�c���ռ���������Ϊ����ƿ��
�ʴ�Ϊ���ƾ��ơ�����ƿ��
��2��Bװ�����ڹ����Һ�巴Ӧ������Ҫ��������ȡ����Ļ�ѧ��Ӧ��ʵ�������������ù���������Һ�ڶ������̵Ĵ��·�Ӧ����������ˮ��
��ȡ������̼�ķ�Ӧ���Ǵ���ʯ����ʯ��ʯ����ϡ���ᣬ��Ӧ�����dz��£����Է�Ӧ���ǹ����Һ�巴Ӧ�������Dz���Ҫ���ȣ�����װ��Ӧѡ��B��
�ʴ�Ϊ��2H202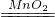 2H20+O2���� B�� CaCO3+2HCl�TCaCl2+CO2��+H2O��
2H20+O2���� B�� CaCO3+2HCl�TCaCl2+CO2��+H2O��
��3����������֪�����÷�Ӧ�ķ�Ӧ������ˮ�����ƺͼ�ʯ�ҵĹ��������Ӧ�����Ǽ��ȣ����Է���װ����ѡ��A�����ɵļ������ܶ�С�ڿ�����������ˮ�����壬����Ӧѡ�������ſ���������ˮ����
�ʴ�Ϊ��A��D��E��CH4+202 C02+2H20
C02+2H20
��������1���������������ƺ���;�ش�
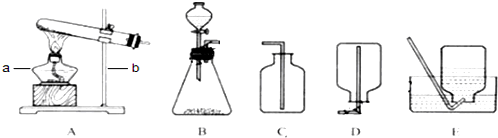
��2�����ݷ�Ӧ���״̬�ͷ�Ӧ������ѡ��������ķ���װ�ã�Bװ�����ڹ����Һ�巴Ӧ������Ҫ��������ȡ����Ļ�ѧ��Ӧ��
��3���ٸ��ݷ�Ӧ���״̬�ͷ�Ӧ������ѡ��������ķ���װ�ã��ڸ��������ˮ���Ժ��ܶȵĴ�С��ѡ����������ռ�װ�ã�
�������˽ⳣ��������ʹ�ã����ճ�������ķ������ռ�װ�ü�ѡȡ��������ȷ��д��ѧ����ʽ��
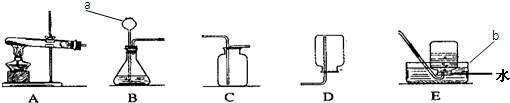
��1��aΪ��Դ�������оƾ��ƣ�c���ռ���������Ϊ����ƿ��
�ʴ�Ϊ���ƾ��ơ�����ƿ��
��2��Bװ�����ڹ����Һ�巴Ӧ������Ҫ��������ȡ����Ļ�ѧ��Ӧ��ʵ�������������ù���������Һ�ڶ������̵Ĵ��·�Ӧ����������ˮ��
��ȡ������̼�ķ�Ӧ���Ǵ���ʯ����ʯ��ʯ����ϡ���ᣬ��Ӧ�����dz��£����Է�Ӧ���ǹ����Һ�巴Ӧ�������Dz���Ҫ���ȣ�����װ��Ӧѡ��B��
�ʴ�Ϊ��2H202
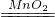 2H20+O2���� B�� CaCO3+2HCl�TCaCl2+CO2��+H2O��
2H20+O2���� B�� CaCO3+2HCl�TCaCl2+CO2��+H2O����3����������֪�����÷�Ӧ�ķ�Ӧ������ˮ�����ƺͼ�ʯ�ҵĹ��������Ӧ�����Ǽ��ȣ����Է���װ����ѡ��A�����ɵļ������ܶ�С�ڿ�����������ˮ�����壬����Ӧѡ�������ſ���������ˮ����
�ʴ�Ϊ��A��D��E��CH4+202
 C02+2H20
C02+2H20��������1���������������ƺ���;�ش�
��2�����ݷ�Ӧ���״̬�ͷ�Ӧ������ѡ��������ķ���װ�ã�Bװ�����ڹ����Һ�巴Ӧ������Ҫ��������ȡ����Ļ�ѧ��Ӧ��
��3���ٸ��ݷ�Ӧ���״̬�ͷ�Ӧ������ѡ��������ķ���װ�ã��ڸ��������ˮ���Ժ��ܶȵĴ�С��ѡ����������ռ�װ�ã�
�������˽ⳣ��������ʹ�ã����ճ�������ķ������ռ�װ�ü�ѡȡ��������ȷ��д��ѧ����ʽ��

��ϰ��ϵ�д�
�����Ŀ