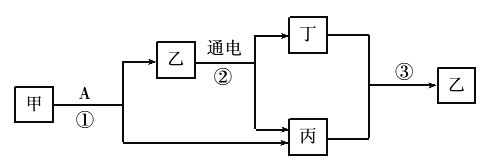
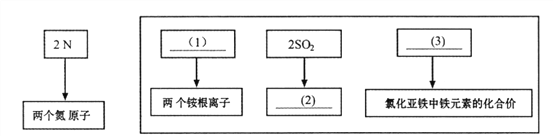
��Ŀ����
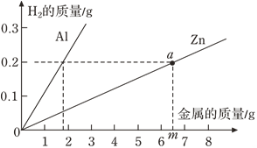
����Ŀ��ijУ��ѧ��ȤС���ڲμ����ʵ���ʱ���������һʪ��ұͭ���ŷŵķ�ˮ�к������������ͭ������Ⱦ�Ϊ�ⶨ�÷�ˮ�и���Ⱦ��ĺ�������ұͭ���ṩ������ˮ�IJο����������ͬѧ����������ʵ�飺ȡ��ˮ300g�������м���������������Ϊ20%������������Һ����ó��������������������������Һ��������ϵ��ͼ����������㣺
(1)ʵ��������������ͭ������Ϊ______g��
(2)������ͭ��Ӧ������������Һ������_________________��
(3)�÷�ˮ�������������������______________��(��������ȷ��0.1%)
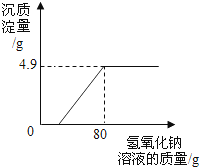
���𰸡�4.9g 20 4.9%
��������
�⣺(1)����ͼ���֪������������ͭ������������4.9g��
(2)���ˮ�м�������������Һ������������Һ�����ˮ�е�ϡ���ᷴӦ��2NaOH+H2SO4==Na2SO4+2H2O����ϡ���ᷴӦ���Ժ�����������Һ��������ͭ��Ӧ��2NaOH+CuSO4 ==Cu(OH)2��+Na2SO4 ����300g�÷�ˮ������ͭ������Ϊx.
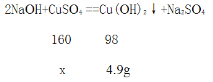
![]() =
=![]() �����X=8g
�����X=8g
��������ͭ��Ӧ������������Һ��������y��
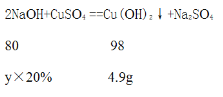
![]() =
=![]() �����y=20g
�����y=20g
(3)����ͼ���֪����Ӧ����ȥ����������Һ80g��������ϡ���ᷴӦ������������Һ������Ϊ80g-20g=60g
���300g��ˮ�к����������Ϊz��
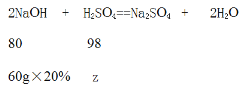
![]() =
=![]() �����z=14.7g
�����z=14.7g
�÷�ˮ�������������������Ϊ��![]() ��100%=4.9%
��100%=4.9%

 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д�