��Ŀ����
������ʵ���ҳ��õļ���������

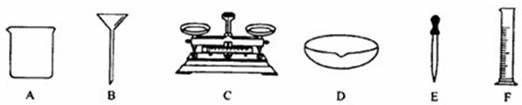
��1��д��������ȡ�͵μ�����Һ����������_________��
��2����ȥ����������Һ�л��е�������������ͭ��Һ��ͨ�����������_________����Ӧ�Ļ�ѧ����ʽ_________����ַ�Ӧ����ˣ��������õ����������е�_________�����ţ���
��3������500g������������Ϊ0.5%�ĸ��������Һ�����ӽ����������������ص�����Ϊ_________����ȡҩƷʱ�õ���������_________�����ţ�����ȷ�Ƶ����������غ���ȡ����ˮʱ�������Ӷ�����������õ���Һ������������������_________����ƫ��ƫС����
��2����ȥ����������Һ�л��е�������������ͭ��Һ��ͨ�����������_________����Ӧ�Ļ�ѧ����ʽ_________����ַ�Ӧ����ˣ��������õ����������е�_________�����ţ���
��3������500g������������Ϊ0.5%�ĸ��������Һ�����ӽ����������������ص�����Ϊ_________����ȡҩƷʱ�õ���������_________�����ţ�����ȷ�Ƶ����������غ���ȡ����ˮʱ�������Ӷ�����������õ���Һ������������������_________����ƫ��ƫС����
��1��E
��2�����ۣ�Fe+CuSO4==Cu+FeSO4��B
��3��2.5g��C��ƫ��
��2�����ۣ�Fe+CuSO4==Cu+FeSO4��B
��3��2.5g��C��ƫ��

��ϰ��ϵ�д�
 �ƸԾ���Ȥζ����ϵ�д�
�ƸԾ���Ȥζ����ϵ�д� ����С����ҵ��ϵ�д�
����С����ҵ��ϵ�д�
�����Ŀ