��Ŀ����
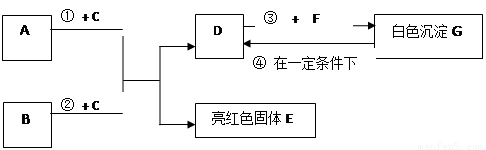
��9�֣�A~G���dz��л�ѧѧϰ���ij������ʣ���Щ������һ�������£�����������ͼ��ʾ��ת����ϵ����֪A��EΪ���ʣ�����Ϊ�������G�Ǵ���ʯ�е���Ҫ�ɷ֡�
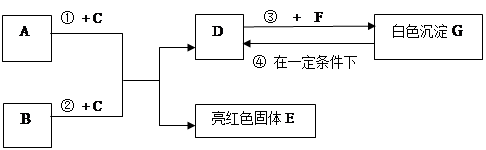 |
�����ͼʾ�Ʋ⣬�ش��������⣺
���£���������ɱ�����Һ�ķ��� B B. ���루1��B��C�Ļ�ѧʽ�ֱ��� �� ��
��2��F��ˮ��Һ�������������� ��
��3����Ӧ�� �Ļ�ѧ����ʽΪ_____________ ��
��4���ڳ����·����ķ�Ӧ�ܵĻ�ѧ����ʽΪ __________________ ��
��5��50g G��һ��������ת��ΪDʱ�����õ���D�������� g��
��1�� CO ��1�֣� CuO ��1�֣�
��2�� Ca2+��OH�C ��1�֣�
��3�� C + 2CuO  2CuO + CO2�� ��2�֣�δ��ƽ��©������©�� ֻ��1�֣�
2CuO + CO2�� ��2�֣�δ��ƽ��©������©�� ֻ��1�֣�
��4�� CaCO3 + 2HCl = CaCl2 + H2O + CO2�� ��2�֣�δ��ƽ��©�� ֻ��1�֣�
��5�� 22 ��2�֣�
����

��9�֣�A~G���dz��л�ѧѧϰ���ij������ʣ���Щ������һ�������£�����������ͼ��ʾ��ת����ϵ����֪A��EΪ���ʣ�����Ϊ�������G�Ǵ���ʯ�е���Ҫ�ɷ֡�
 |
�����ͼʾ�Ʋ⣬�ش��������⣺
��1��B��C�Ļ�ѧʽ�ֱ��� �� ��
��2��F��ˮ��Һ�������������� ��
��3����Ӧ�� �Ļ�ѧ����ʽΪ_____________ ��
��4���ڳ����·����ķ�Ӧ�ܵĻ�ѧ����ʽΪ __________________ ��
��5��50g G��һ��������ת��ΪDʱ�����õ���D�������� g��
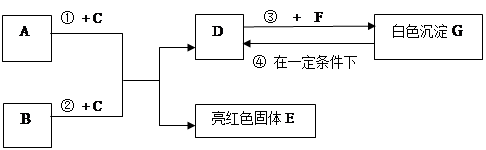 |
�����ͼʾ�Ʋ⣬�ش��������⣺
���£���������ɱ�����Һ�ķ��� B B. ���루1��B��C�Ļ�ѧʽ�ֱ��� �� ��
��2��F��ˮ��Һ�������������� ��
��3����Ӧ�� �Ļ�ѧ����ʽΪ_____________ ��
��4���ڳ����·����ķ�Ӧ�ܵĻ�ѧ����ʽΪ __________________ ��
��5��50g G��һ��������ת��ΪDʱ�����õ���D�������� g��
��9�֣�A~G���dz��л�ѧѧϰ���ij������ʣ���Щ������һ�������£�����������ͼ��ʾ��ת����ϵ����֪A��EΪ���ʣ�����Ϊ�������G�Ǵ���ʯ�е���Ҫ�ɷ֡�
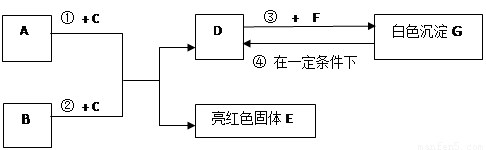 |
�����ͼʾ�Ʋ⣬�ش��������⣺
��1��B��C�Ļ�ѧʽ�ֱ��� �� ��
��2��F��ˮ��Һ�������������� ��
��3����Ӧ�� �Ļ�ѧ����ʽΪ_____________ ��
��4���ڳ����·����ķ�Ӧ�ܵĻ�ѧ����ʽΪ __________________ ��
��5��50g G��һ��������ת��ΪDʱ�����õ���D�������� g��