��Ŀ����
ij�߸�Ƭ�ı�ǩ��ͼ������ϸ�Ķ���ش��������⣺��1��ά����D����϶࣬����ά����D2�Ļ�ѧʽΪC28H44O���Լ��㣺
��ά����D2��̼ԭ�ӡ���ԭ�ӵĸ����ȣ�______
��ά����D2���⡢��Ԫ�ص������ȣ�______
��2��ijͬѧҪ̽���ø�Ƭ����Ҫ�ɷ�̼��Ƶĺ����Ƿ�ȷ����ȡ��10Ƭ��Ƭ������������ϡ���ᣬ��ַ�Ӧ���ռ���4.4g������̼���ٶ������ɷֲ������ᷴӦ����ͨ�������жϸø߸�Ƭ�Ƿ�Ϊ�ϸ�ҩƷ��Ҫ�м�����̣���

���𰸡���������1����Է��������Ǹ�Ԫ��ԭ����֮�ͣ�Ԫ�ص��������Ǹ�Ԫ��ԭ�����͵ı�ֵ
��2�����ö�����̼����������̼��Ƶ��������ٽ��Ҫ������жϼ��ɣ�
����⣺
��1����ά����D2��̼ԭ�ӡ���ԭ�ӵĸ�����=28��44=7��11
��ά����D2���⡢������Ԫ�ص��������ǣ�1×44��16=11��4
��2����10Ƭ��̼��Ƶ�����Ϊx��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 4.4g

X=10g
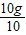 =1g��1.5g
=1g��1.5g
���Ըø߸�Ƭ�Dz��ϸ�ҩƷ��
�ʴ�Ϊ��
��1����7��11����11��4��
��2�����Ըø߸�Ƭ�Dz��ϸ�ҩƷ��
���������ʵĻ�ѧʽ���Ա�ʾ���ʵ���ɼ����ʵķ��ӹ��ɣ����Ը������ʵĻ�ѧʽ������Է������������Ԫ�������ȡ����Ԫ�����������ȵļ��㣮
��2�����ö�����̼����������̼��Ƶ��������ٽ��Ҫ������жϼ��ɣ�
����⣺
��1����ά����D2��̼ԭ�ӡ���ԭ�ӵĸ�����=28��44=7��11
��ά����D2���⡢������Ԫ�ص��������ǣ�1×44��16=11��4
��2����10Ƭ��̼��Ƶ�����Ϊx��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 4.4g

X=10g
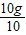 =1g��1.5g
=1g��1.5g���Ըø߸�Ƭ�Dz��ϸ�ҩƷ��
�ʴ�Ϊ��
��1����7��11����11��4��
��2�����Ըø߸�Ƭ�Dz��ϸ�ҩƷ��
���������ʵĻ�ѧʽ���Ա�ʾ���ʵ���ɼ����ʵķ��ӹ��ɣ����Ը������ʵĻ�ѧʽ������Է������������Ԫ�������ȡ����Ԫ�����������ȵļ��㣮

��ϰ��ϵ�д�
 ��һ����ͬ���ɽ�����ϵ�д�
��һ����ͬ���ɽ�����ϵ�д�
�����Ŀ
 ij�߸�Ƭ�ı�ǩ��ͼ������ϸ�Ķ���ش��������⣺
ij�߸�Ƭ�ı�ǩ��ͼ������ϸ�Ķ���ش��������⣺ ij�߸�Ƭ�ı�ǩ��ͼ������ϸ�Ķ���ش��������⣺
ij�߸�Ƭ�ı�ǩ��ͼ������ϸ�Ķ���ش��������⣺
