��Ŀ����
����Ŀ����13�֣�������ʵ������ȡ���ռ������װ�ã�������ѧ֪ʶ�ش��������⣺
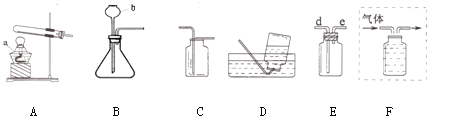
��1��д�����������ƣ�a ��b
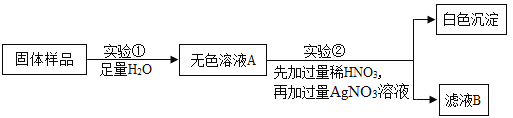
��2������ͼ��ѡ��װ����ȡ���ռ������CO2��Ӧѡ��ͼ�е� ������ĸ����
��3��ʵ��������װ��E�ռ�H2������Ӧ�ӵ��ܵ� ������d������e�����˽��롣
��4������ȡ��O2��Bװ����ȡ�ķ�Ӧԭ�� ����Fװ����ˮ���ռ����뽫ͼF����������ֱ����ͼ�л�������
��5����ѧ��ȤС��Ϊ�˲ⶨʯ��ʯ��Ʒ��̼��Ƶ�����������ȡ25gʯ��ʯ��Ʒ�����ձ��У�Ȼ����������μ���һ����ij����������ϡ���ᣬʹ֮����Ʒ��ַ�Ӧ�����ʲ��μӷ�Ӧ�������ŷ�Ӧ���У�����ϡ����������뷴Ӧ�õ�����������仯��ϵ��ͼ��ʾ����������м������ݣ���д��������̣�

����Ʒ��̼��Ƶ����������Ƕ��٣�
������ϡ�������ʵ����������Ƕ��٣�
���𰸡���1���ƾ��� ����©�� ��2��BEC ��3��e
��4��2H2O2![]() 2H2O + O2�� ���Ҷ˵ĵ����ӳ�������ƿ�ײ�
2H2O + O2�� ���Ҷ˵ĵ����ӳ�������ƿ�ײ�
��5����80% ��10%
�������������������1������������ʶ�ǣ�a���ƾ��� b������©��
��2�����巢��װ�õ�ѡ�����ݣ���Ӧ���״̬�ͷ�Ӧ��������ȡCO2�Dz��ù������ʯ��Һ��ϡ�����ڳ����·�Ӧ����ѡ����װ��B���ռ�װ�õ�ѡ�����ݣ�������ܶȺ��ܽ��ԣ����ڶ�����̼���ܶȱȿ�������������ˮ����ѡ�ռ�װ��C ���ټ���Ҫ����ʻ���ѡ E
��3�������������ܶȱȿ���С������װ��E�ռ�H2������Ӧ�ӵ��ܵ�e�˽���
��4������ȡ��O2��Bװ����ȡ��˵���Dz��ù���������Һ�Ͷ������̻�ϣ���Ӧԭ����2H2O2MnO22H2O + O2������Fװ����ˮ���ռ����̶��Ӷ̹ܽ�������ֻ�轫�������ӳ���ƿ���ɣ�ͼ��
��5������ͼ���֪������Ӧ���������Ķ�����̼��������Ϊ8.8g�����ݻ�ѧ��Ӧ����ʽ��CaCO3+ 2HCl ="=" CaCl2+ H2O + CO2����CO2��CaCO3��HCl��������ϵ���ɷֱ����CO3��HCl����������һ���ɷֱ������Ʒ��̼��Ƶ���������������ϡ�������ʵ���������
�⣺��̼��Ƶ�����Ϊx��HCl������Ϊy
CaCO3+ 2HCl ="=" CaCl2+ H2O + CO2��
100 73 44
x y 8.8g
��100��44=x��8.8g x��20g
��Ʒ��̼��Ƶ���������=20g/25g��100%��80%
�� 73:44=y:8.8g y��14.6g
����ϡ�������ʵ���������=14.6g/146��100%��10%
����