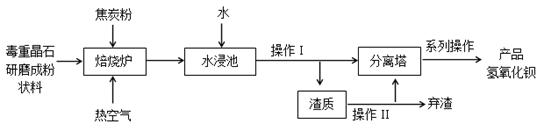
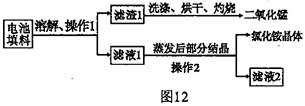
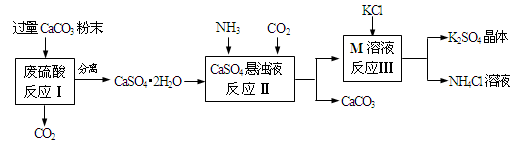
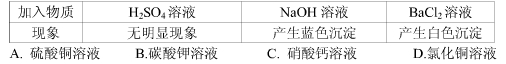
��Ŀ����
��3�֣��������ܴ�ˮ���ܴ���ˮ���٣���ˮ���ܽ��˺ܶ����ʣ��ֿ����̣����˺�H2O�⣬�����д�����Na+ ��Ca2+��Cl-��Mg2+��SO42- �ȡ��Ժ�ˮΪԭ����ȡʳ�εĹ����������£�
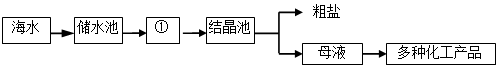
��1��ͼ�Т��� �أ������������ȴ������
��2�����ݺ�ˮɹ�ε�ԭ��������˵���в���ȷ���� ��
A�� �õ��Ĵ����Ǵ������Ȼ���
B�� �ڢ��У���ˮ��ˮ����������
C�� �ڢ��У���ˮ���Ȼ��Ƶ�����������
D�� ��ˮ������ˮ�أ���ˮ�ijɷֻ�������
��3��������������������ͼ��ĸҺ�������������� ��
A�� Ca2+��Mg2+��SO42-�� B�� Na+ ��Ca2+��Cl-��Mg2+��SO42-��H2O��
C�� Ca2+��Mg2+��SO42-��H2O�� D�� Na+ ��Ca2+��Cl-��Mg2+��SO42-��

��1��ͼ�Т��� �أ������������ȴ������
��2�����ݺ�ˮɹ�ε�ԭ��������˵���в���ȷ���� ��
A�� �õ��Ĵ����Ǵ������Ȼ���
B�� �ڢ��У���ˮ��ˮ����������
C�� �ڢ��У���ˮ���Ȼ��Ƶ�����������
D�� ��ˮ������ˮ�أ���ˮ�ijɷֻ�������
��3��������������������ͼ��ĸҺ�������������� ��
A�� Ca2+��Mg2+��SO42-�� B�� Na+ ��Ca2+��Cl-��Mg2+��SO42-��H2O��
C�� Ca2+��Mg2+��SO42-��H2O�� D�� Na+ ��Ca2+��Cl-��Mg2+��SO42-��
��1������ ��2��AC ��3��D
�����������1���Ȼ��Ƶ��ܽ�����¶ȵ����߶��仯���ʴӺ�ˮ����ȡ�Ȼ���Ӧ��ȡ�����ᾧ�ķ���
��2��A���õ��Ĵ�������Ҫ���Ȼ��ƵĻ�������B���ڢ��У��Dz�������ˮ�ֵķ��������Ժ�ˮ��ˮ���������٣���ȷ��C���ڢ��У�������ˮ�ֵĹ����У���ˮ���Ȼ��Ƶ�����Ӧһֱ���䣬����D����ˮ������ˮ�أ���ˮ�ijɷֻ������䣬��ȷ����ѡA��C
��3����Ϊ��ˮ���ܽ��˺ܶ����ʣ��ֿ����̣����˺�H2O�⣬�����д�����Na+ ��Ca2+��Cl-��Mg2+��SO42- �ȣ���ͨ������ˮ�֣�ֻ�Ǵֵ��Ȼ��ƽᾧ����������ĸҺ��������������Ȼ�У�Na+ ��Ca2+��Cl-��Mg2+��SO42-�ȣ�ѡD

��ϰ��ϵ�д�
 ��һ����ͬ���ɽ�����ϵ�д�
��һ����ͬ���ɽ�����ϵ�д� ������Ӧ���ϵ�д�
������Ӧ���ϵ�д� ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�
�����Ŀ