��Ŀ����
�±��г�����������Ħ�����������������
��1��
��2����������Ʋ⣬����Ħ�������ܽ����� ������ܡ������ܡ���
��3�������е�Ħ����֮һ̼��ƿ�����ʯ��ʯ���Ʊ���ijѧ�������һ���Ʊ�̼��Ƶ�ʵ�鷽����������ͼΪ
ʯ��ʯ
��ʯ��
ʯ��ˮ
̼���
��д�������������йط�Ӧ�Ļ�ѧ����ʽ��
�� ��
�� ��
�� ��
��4����������ʯ��ʯΪԭ�ϣ������Լ���ѡ���������һ���Ʊ�̼��Ƶ�ʵ�鷽���������գ�3����ʾ����ͼ��������ͼ�е������������ʵ�������
ʯ��ʯ
��ʯ��
ʯ��ˮ
̼���
����Ƶķ������ŵ��� ��
��5�������������Ƿ���̼���ε�ʵ�鷽���� ��
��1��
| ���������� | ���������� | ����� | |
| Ħ���� | �������� | ̼��� | �������� |
| Ħ������������� ��ָ�ᡢ��Ρ������ |
��3�������е�Ħ����֮һ̼��ƿ�����ʯ��ʯ���Ʊ���ijѧ�������һ���Ʊ�̼��Ƶ�ʵ�鷽����������ͼΪ
ʯ��ʯ
| ���� |
| ��ˮ |
| ��̼������Һ |
��д�������������йط�Ӧ�Ļ�ѧ����ʽ��
��
��
��
��4����������ʯ��ʯΪԭ�ϣ������Լ���ѡ���������һ���Ʊ�̼��Ƶ�ʵ�鷽���������գ�3����ʾ����ͼ��������ͼ�е������������ʵ�������
ʯ��ʯ
| ���� |
| () |
| () |
����Ƶķ������ŵ���
��5�������������Ƿ���̼���ε�ʵ�鷽����
������̼����ֽܷ����������ƣ�����������ˮ��Ӧ�����������ƣ������������������̼��̼���Ʒ�Ӧ����̼��ƣ�����̼����ͨ��ʹ�ü��ữ���ķ�����
����⣺��1�������������ڼ̼��������Σ���������������������Ա����Ϊ��
��2������������̼��ơ��������趼��������ˮ�����ʣ����Ա����Ϊ�����ܣ�
��3��̼����ܸ��·ֽ����������ƺͶ�����̼������������ˮ��Ӧ�����������ƣ�������������̼���Ʒ������ֽⷴӦ����̼��ƺ��������ƣ����Ա����Ϊ��
��CaCO3
CaO+CO2����
��CaO+H2O�TCa��OH��2��
��Ca��OH��2+Na2CO3�TCaCO3��+2NaOH��
��4��̼��ƻ����������������������̼��Ӧ���ɣ���ʱ��������̼��Ʒֽ����ɵĶ�����̼���ﵽ��Լ��Ŀ�ģ����Ա����Ϊ��
������̼�õ�������ã���Լԭ�ϣ�
��5������̼���γ�ʹ�ü��ữ���ķ������۲�����������ܷ�ʹʯ��ˮ����ǣ����Ա����Ϊ���������м���ϡ���ᣬ���Ƿ��������������������ų������ɵ�������ʹ�����ʯ��ˮ����ǣ��������к���̼���Σ�����̼���Σ�
| ���������� | �������������� | ������� | |
| �� | �� | ������ |
��3��̼����ܸ��·ֽ����������ƺͶ�����̼������������ˮ��Ӧ�����������ƣ�������������̼���Ʒ������ֽⷴӦ����̼��ƺ��������ƣ����Ա����Ϊ��
��CaCO3
| ||
��CaO+H2O�TCa��OH��2��
��Ca��OH��2+Na2CO3�TCaCO3��+2NaOH��
��4��̼��ƻ����������������������̼��Ӧ���ɣ���ʱ��������̼��Ʒֽ����ɵĶ�����̼���ﵽ��Լ��Ŀ�ģ����Ա����Ϊ��

������̼�õ�������ã���Լԭ�ϣ�
��5������̼���γ�ʹ�ü��ữ���ķ������۲�����������ܷ�ʹʯ��ˮ����ǣ����Ա����Ϊ���������м���ϡ���ᣬ���Ƿ��������������������ų������ɵ�������ʹ�����ʯ��ˮ����ǣ��������к���̼���Σ�����̼���Σ�
���������⿼����̼��ơ������ƺ��������Ƶ��ת���Լ�̼���εļ��飬��ɴ��⣬�����������е�֪ʶ���У�

��ϰ��ϵ�д�
 ����������������ϵ�д�
����������������ϵ�д�
�����Ŀ
�������̽��Ҫ�õ����ѧ֪ʶ��
��1���±��г�����������Ħ�����������������
��2����������Ʋ⣬����Ħ�������ܽ����� ������ܡ������ܡ�����
��3�������е�Ħ����֮һ̼��ƿ�����ʯ��ʯ���Ʊ���ijѧ�������һ���Ʊ�̼��Ƶ�ʵ�鷽����������ͼΪ��

��д�������������йط�Ӧ�Ļ�ѧ����ʽ��
�� ���� ���� ��
��4����������ʯ��ʯΪԭ�ϣ������Լ���ѡ���������һ���Ʊ�̼��Ƶ�ʵ�鷽�������գ�3����ʾ�������ʵ�鷽��������ͼ��ʾ������
����Ƶķ����ŵ��� ��
��5�������������Ƿ���̼���ε�ʵ�鷽���� ��
��1���±��г�����������Ħ�����������������
| ���������� | �������������� | ������� | |
| Ħ���� | �������� | ̼��� | �������� |
| Ħ�������������ָ�ᡢ��Ρ������ |
��3�������е�Ħ����֮һ̼��ƿ�����ʯ��ʯ���Ʊ���ijѧ�������һ���Ʊ�̼��Ƶ�ʵ�鷽����������ͼΪ��

��д�������������йط�Ӧ�Ļ�ѧ����ʽ��
��
��4����������ʯ��ʯΪԭ�ϣ������Լ���ѡ���������һ���Ʊ�̼��Ƶ�ʵ�鷽�������գ�3����ʾ�������ʵ�鷽��������ͼ��ʾ������
����Ƶķ����ŵ���
��5�������������Ƿ���̼���ε�ʵ�鷽����
�������̽��Ҫ�õ����ѧ֪ʶ��
��1���±��г�����������Ħ�����������������
| ���������� | �������������� | ������� | |
| Ħ���� | �������� | ̼��� | �������� |
| Ħ�������������ָ�ᡢ��Ρ������ |
��3�������е�Ħ����֮һ̼��ƿ�����ʯ��ʯ���Ʊ���ijѧ�������һ���Ʊ�̼��Ƶ�ʵ�鷽����������ͼΪ��

��д�������������йط�Ӧ�Ļ�ѧ����ʽ��
��________����________����________��
��4����������ʯ��ʯΪԭ�ϣ������Լ���ѡ���������һ���Ʊ�̼��Ƶ�ʵ�鷽�������գ�3����ʾ�������ʵ�鷽��������ͼ��ʾ������
����Ƶķ����ŵ���________��
��5�������������Ƿ���̼���ε�ʵ�鷽����________��
СԶͨ����������֪�����������Ҫ�ɷ֣�����һ��������̼��Ʒ�ĩ��ʳ�ε���С�ձ��У�Ȼ������������ͣ���ʪ��������ζ�����㾫�ȣ�������Ⱥ��Ƶ����࣮
��1��������______������������
��2��СԶ�ⶨ�������༰���������Ʒ��pH����¼���£�
����������______������ԡ��������ԡ��������ԡ�����������ʹ��ɫʯ����Һ���______ɫ��
��3���±��г�����������Ħ�����������������
��4�������е�Ħ����֮һ̼��ƿ�����ʯ��ʯ���Ʊ���ijѧ�������һ���Ʊ�̼��Ƶ�ʵ�鷽����������ͼΪ��
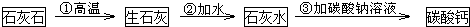
��д�������������йط�Ӧ�Ļ�ѧ����ʽ��
��______ CaO+CO2��
��1��������______������������
��2��СԶ�ⶨ�������༰���������Ʒ��pH����¼���£�
| ���� | �������� | ����� | ���۾� |
| pH | 8 | 2 | 12 |
��3���±��г�����������Ħ�����������������
| ���������� | ���������� | ����� | |
| Ħ���� | �������� | ̼��� | �������� |
| Ħ������������� ��ָ�ᡢ��Ρ������ | ______ | ______ | ______ |
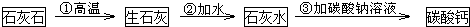
��д�������������йط�Ӧ�Ļ�ѧ����ʽ��
��______ CaO+CO2��
