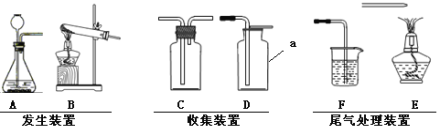
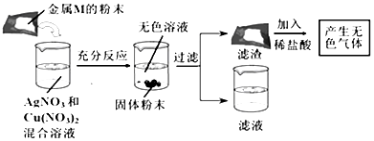
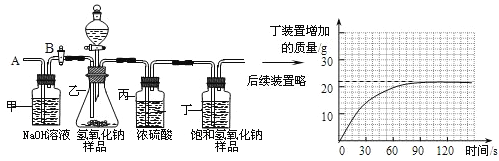
��Ŀ����
����Ŀ��(4����Ϊ�����о����ڷ�����������ҩƷ�����������ѧ��ȤС���ͬѧ���������ʵ�飬����Ҫʵ�鲽�����£�
��ͼ��װ��������55��0g����������ƿ�У���������ϡ������Һ��
����һ��ʱ����װ�ö����������ӵ���������������������
����ƿ�в��ٲ�������ʱ������B���ӵ���A����������һ�����Ŀ�����
�����ٴγ���װ�ö����������ӵ�������
�����ظ���͢��IJ�����ֱ��װ�ö��������������䣮
�����ʵ�����ݣ�ͨ����������������Ʊ����������ȫ���ʡ��������ֱ��ʡ������ʡ���Ҫ����ϸ�ļ��㲽�裬û�в��費�÷���
���𰸡��������Ʋ��ֱ��ʡ�
��������
������������ݶ�����̼���������û�ѧ����ʽ���м��㡣
�⣺���ݱ����֪���ɶ�����̼������Ϊ22g (1����
��55��0g����������Ʒ��̼��������Ϊx
H2SO4+Na2CO3=Na2SO4+H2O+CO2�� (1����
106 44
x 22g
![]()
x=53g (1����
53g��55��0g
���������Ʋ��ֱ���
���������Ʋ��ֱ����� (1����

��ϰ��ϵ�д�
�����Ŀ