��Ŀ����
13����A��B��C��D�������ʣ���ͼ��ʾ��A��B��C��һ���¿��Է���ת������C��Һ��ͨ��CO2����Һ����ǣ����ɰ�ɫ����A��D��A��B��C���ܷ�����Ӧ��D��C�����кͷ�Ӧ��D��A��Ӧ��CO2���������D��AgNO3��Һ��Ӧ���ɲ���������ϡ����İ�ɫ������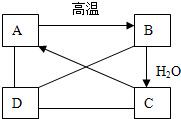
��1���������֪�����ƶ��������ʵĻ�ѧʽ��
A
C
��2����д�����з�Ӧ�Ļ�ѧ����ʽ��
C��Һ��ͨ��CO2����Һ�����
B����ˮ�ų�������������C

��1���������֪�����ƶ��������ʵĻ�ѧʽ��
A
CaCO
BCaO
C
Ca��OH��2
DHCl
��2����д�����з�Ӧ�Ļ�ѧ����ʽ��
C��Һ��ͨ��CO2����Һ�����
Ca��OH��2+CO2�TCaCO3��+H2O
B����ˮ�ų�������������C
CaO+H2O�TCa��OH��2
��D��A��ӦCaCO3+2HCl�TCaCl2+H2O+CO2��
��������������������Ӧ���ɲ���������İ�ɫ�������ó���ֻ�����Ȼ�����D��C�����кͷ�Ӧ��D��A��Ӧ��CO2���������Dֻ�����ᣬͨ�������̼���ɵİ�ɫ������̼��ƣ�
����⣺������һ���ƶ��⣮�ƶ���ķ�����Ѱ�ҡ����ۡ�����Ŀ�С���C��Һ��ͨ��CO2����Һ����ǣ����ɰ�ɫ����A��֪AΪCaCO3��CΪCa��OH��2����ͼ��֪A�ڸ���������������B��B������ˮ��Ӧ����C�����ƶ�BΪCaO������ϵ��Ŀ�С�D��A��B��C���ܷ�����Ӧ��D��C�����кͷ�Ӧ��D��A��Ӧ��CO2���������D��AgNO3��Һ��Ӧ���ɲ���������ϡ����İ�ɫ������֪�ɷ���DΪHCl��
�ʴ�Ϊ����1��A��CaCO3B��CaOC��Ca��OH��2D��HCl
��2��Ca��OH��2+CO2�TCaCO3��+H2O��
CaO+H2O�TCa��OH��2
CaCO3+2HCl�TCaCl2+H2O+CO2��
�ʴ�Ϊ����1��A��CaCO3B��CaOC��Ca��OH��2D��HCl
��2��Ca��OH��2+CO2�TCaCO3��+H2O��
CaO+H2O�TCa��OH��2
CaCO3+2HCl�TCaCl2+H2O+CO2��
���������������ƶ���Ĺ����У���Ҫ�������ͻ�Ƶ㣬�����ͻ�Ƶ���Dz���������İ�ɫ��������C��ͨ��CO2����Һ����ǣ����ɰ�ɫ����A��

��ϰ��ϵ�д�
�����Ŀ