��Ŀ����
��������ͭ��ͭ�Ļ�������ɷֽ��з�����ȡ20g����Ʒ�������з���μ�����ͬ����������������ϡ���ᣬʹ֮��ַ�Ӧ��ÿ������ϡ�����������ʣ������������¼���±�������Ӧ�Ļ�ѧ����ʽ��CuO+H2SO4=CuSO4+H2O��
| ��һ�� | �ڶ��� | ������ | ���Ĵ� | ����� | |
| ����ϡ���������/g | 40 | 40 | 40 | 40 | 40 |
| ��ַ�Ӧ��ʣ���������/g | 16 | 12 | 8 | 6 | m |
��1������������m��ֵΪ________��
��2��20g��Ʒ��CuO����������Ϊ________��
��3������������ϡ��������������������Ƕ��٣�
�⣺��1������ϡ�����������ӣ������������ͭ���ϼ��٣�ʣ����������������ϼ�С��������ͭ��ȫ��Ӧ��ʣ�����Ϊͭ���ܼ��������ᷴӦ���������ٸı䣻�ɵ����Ρ����Ĵ�ʵ�����ݿ�֪����ʱ��ʣ�����ȫ��Ϊͭ������������ϡ���ᷢ����Ӧ����˹����������䣻���ԣ�����μ���ϡ���ᣬ����������Ϊ6g��
��2����Ʒ������ͭ������=20g-6g=14g��
CuO����������Ϊ=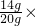 100%=70%��
100%=70%��
��3������ʵ�����ݣ�ǰ����ʵ����ÿ����40gϡ���ᣬʣ�������������4g��˵��������ʵ��ʱ����ϡ������ȫ��Ӧ��ÿ����������ͭ������Ϊ4g��
��������ϡ�������������������Ϊx��������ã�
CuO+H2SO4=CuSO4+H2O
80 98
20g-16g 40g?x
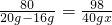
x=12.25%
������ϡ�������������������Ϊ12.25%��
�ʴ�Ϊ��
��1��6
��2��70%
��3����������ϡ�������������������Ϊx��������ã�
CuO+H2SO4=CuSO4+H2O
80 98
20g-16g 40g?x
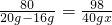
x=12.25%
������ϡ�������������������Ϊ12.25%��
���������ݽ����Ļ�Կ�֪������ͭ��ͭ�Ļ������ͭ������ϡ���ᷢ����Ӧ��������ͭ��ϡ���ᷴӦ��������ͭ��Һ��ˮ�������ϡ����ļ�������������ٵ���Ϊ����ͭ��������
���������ʷ�Ӧ��ȫ����ʣ������������Ϊ������в��μӷ�Ӧ��ͭ��������
����ʵ���¼�����ݣ����Ĵμ���ϡ�����ʣ�����������仯С��˵��������������ϡ���Ტû�������ʷ�����Ӧ��������������ͭ����ȫ��Ӧ����ʣ�����ȫ��Ϊͭ����������ӵ�ϡ����Ҳ��������巢����Ӧ�����ԣ�ʣ��������ʲ��䣻
���ݷ�Ӧ�Ļ�ѧ����ʽ���ɲμӷ�Ӧ������ͭ��������������ϡ������ȫ��Ӧʱ��������������ô�ʱϡ������������������Һ�������������ϡ���������ʵ�����������
���������ݹ�������������ϡ���ᷴӦ���������ʣ����������仯��ԭ����ʵ�������й��������ĸı��жϷ�Ӧ�����������Ϸ�Ӧ�Ļ�ѧ����ʽ���ɲμӷ�Ӧ����ͭ����������μӷ�Ӧ�����������ʹ��ϡ������ȫ��Ӧʱ����ϡ�����������������ϡ�������������������
��2����Ʒ������ͭ������=20g-6g=14g��
CuO����������Ϊ=
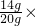 100%=70%��
100%=70%����3������ʵ�����ݣ�ǰ����ʵ����ÿ����40gϡ���ᣬʣ�������������4g��˵��������ʵ��ʱ����ϡ������ȫ��Ӧ��ÿ����������ͭ������Ϊ4g��
��������ϡ�������������������Ϊx��������ã�
CuO+H2SO4=CuSO4+H2O
80 98
20g-16g 40g?x
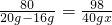
x=12.25%
������ϡ�������������������Ϊ12.25%��
�ʴ�Ϊ��
��1��6
��2��70%
��3����������ϡ�������������������Ϊx��������ã�
CuO+H2SO4=CuSO4+H2O
80 98
20g-16g 40g?x
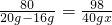
x=12.25%
������ϡ�������������������Ϊ12.25%��
���������ݽ����Ļ�Կ�֪������ͭ��ͭ�Ļ������ͭ������ϡ���ᷢ����Ӧ��������ͭ��ϡ���ᷴӦ��������ͭ��Һ��ˮ�������ϡ����ļ�������������ٵ���Ϊ����ͭ��������
���������ʷ�Ӧ��ȫ����ʣ������������Ϊ������в��μӷ�Ӧ��ͭ��������
����ʵ���¼�����ݣ����Ĵμ���ϡ�����ʣ�����������仯С��˵��������������ϡ���Ტû�������ʷ�����Ӧ��������������ͭ����ȫ��Ӧ����ʣ�����ȫ��Ϊͭ����������ӵ�ϡ����Ҳ��������巢����Ӧ�����ԣ�ʣ��������ʲ��䣻
���ݷ�Ӧ�Ļ�ѧ����ʽ���ɲμӷ�Ӧ������ͭ��������������ϡ������ȫ��Ӧʱ��������������ô�ʱϡ������������������Һ�������������ϡ���������ʵ�����������
���������ݹ�������������ϡ���ᷴӦ���������ʣ����������仯��ԭ����ʵ�������й��������ĸı��жϷ�Ӧ�����������Ϸ�Ӧ�Ļ�ѧ����ʽ���ɲμӷ�Ӧ����ͭ����������μӷ�Ӧ�����������ʹ��ϡ������ȫ��Ӧʱ����ϡ�����������������ϡ�������������������

��ϰ��ϵ�д�
�����Ŀ
| |||||||||||||||