��Ŀ����
ijͬѧ�����һ������ͼ��ʾ��װ�ã����ø�װ�òⶨ��п��Ʒ�ĺ�п����
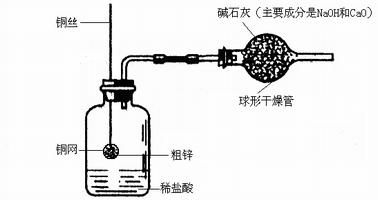
��1������10.0g��п����ͭ���У���ͼ��������װ���ԺƵ�������ҩƷ������Ϊ120.0g��
��2����ͭ����������ϡ�����У���ַ�Ӧ�����Թ۲쵽��ʵ������Ϊ ��
��Ӧ�Ļ�ѧ����ʽΪ ��
��3����Ӧ��ȫ�Ƶ�װ��������Ϊ119.8g�����п�Ĵ���Ϊ ��
��4����ʵ���м�ʯ�ҵ������Ǹ������ͬʱ��ֹϡ����ӷ������Ȼ���������ɢ�������С�
�����ü�ʯ�ң������ⶨ�Ĵ�п���� ���ƫ����ƫС������Ӱ�족��
�����ǿ�����CO2��H2O��ʵ���Ӱ�죩��
��5��������п����ʯ��ʯ��ԭʵ�鷽�� ����ܡ����ܡ�������ʯ��ʯ
��Ʒ���ȵIJⶨ�������� ��
��2���������� Zn��2HCl ![]() ZnCl 2��H2��
ZnCl 2��H2��
��3��65% ��4��ƫ��
��5������ ��ʯ�������ն�����̼��ˮ���������壬���²��ܲ��CO2������

 �żӾ���ϵ�д�
�żӾ���ϵ�д�