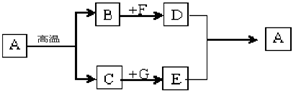
��Ŀ����
������ʹ�����Ľ������ϣ�
��1�������ֶ������Ͻ����к�̼���ϸߵ���________��
��2�����dz��á�ͭǽ���ڡ�����������ļ�̣�������һ��������Ҳ�ܷ������ַ�Ӧ������˿�����ᷴӦ�ų���������Ӧ�Ļ�ѧ����ʽ��________��
��3����mg��������Ϊ10%������ͭ��Һ�м������ۣ���ַ�Ӧ�����ˣ��õ�����A����ҺB���ٽ�����A��������ϡ�����У�������ð������ַ�Ӧ��ʣ��������ʵ�����Ϊ12.8g
�������ijɷ���________����ҺB�ijɷ���________���� ������ͭ��Һ������m=________g��
�⣺��1�������ֶ������Ͻ�������Ǻ�̼����ͬ�����������ĺ�̼���ߣ��ֵĺ�̼���ͣ�
��2���������ᷴӦ�����Ȼ���������������Ӧ�Ļ�ѧ����ʽ��Fe+2HCl=FeCl2+H2����
��3���ڽ����˳����У�������ͭ��ǰ�棬����ͭ���ã��������ܽ�����ͭ��Һ��ͭԪ���û���������ͭ������������������A��������ϡ�����У�������ð����˵������ʣ�࣬���������ijɷ���ͭ����������ͭȫ����Ӧ����������������Һ����ҺB�ijɷ�������������ˮ���ٽ�����A��������ϡ�����У�������ð������ַ�Ӧ����ȫ����Ӧ������ʣ������������ͭ��ͭ������Ϊ12.8g��
������ͭ������Ϊx��
Fe+CuSO4=Cu+FeSO4
160 64
x 12.8g
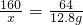
x=32g
����ͭ��Һ������=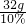 =320g
=320g
������ͭ��Һ������Ϊ320g��
�ʴ�Ϊ����1��������
��2��Fe+2HCl=FeCl2+H2����
��3����Cu��Fe�� FeSO4��H2O�� ��320g��
��������1�����������ֶ������Ͻ�������Ǻ�̼����ͬ���н��
��2�������������ᷴӦ�����Ȼ��������������н��
��3���������ܺ�����ͭ��Ӧ����ͭ������������������A��������ϡ�����У�������ð����˵������ʣ�࣬����ͭȫ����Ӧ��ʣ������������ͭ������������ͭ�������������ͭ�����������������ͭ��Һ���������н��
������������Ҫ���������й����ʣ�����ȷ��д��ѧ����ʽ�����м��㣬�ѶȽϴ�
��2���������ᷴӦ�����Ȼ���������������Ӧ�Ļ�ѧ����ʽ��Fe+2HCl=FeCl2+H2����
��3���ڽ����˳����У�������ͭ��ǰ�棬����ͭ���ã��������ܽ�����ͭ��Һ��ͭԪ���û���������ͭ������������������A��������ϡ�����У�������ð����˵������ʣ�࣬���������ijɷ���ͭ����������ͭȫ����Ӧ����������������Һ����ҺB�ijɷ�������������ˮ���ٽ�����A��������ϡ�����У�������ð������ַ�Ӧ����ȫ����Ӧ������ʣ������������ͭ��ͭ������Ϊ12.8g��
������ͭ������Ϊx��
Fe+CuSO4=Cu+FeSO4
160 64
x 12.8g
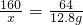
x=32g
����ͭ��Һ������=
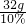 =320g
=320g������ͭ��Һ������Ϊ320g��
�ʴ�Ϊ����1��������
��2��Fe+2HCl=FeCl2+H2����
��3����Cu��Fe�� FeSO4��H2O�� ��320g��
��������1�����������ֶ������Ͻ�������Ǻ�̼����ͬ���н��
��2�������������ᷴӦ�����Ȼ��������������н��
��3���������ܺ�����ͭ��Ӧ����ͭ������������������A��������ϡ�����У�������ð����˵������ʣ�࣬����ͭȫ����Ӧ��ʣ������������ͭ������������ͭ�������������ͭ�����������������ͭ��Һ���������н��
������������Ҫ���������й����ʣ�����ȷ��д��ѧ����ʽ�����м��㣬�ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
 ������ʹ�����Ľ������ϣ�
������ʹ�����Ľ������ϣ�