��Ŀ����
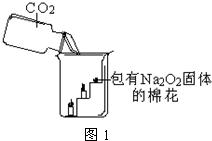
17���������ƣ�Na2O2���ڳ����������˺����Ķ�����̼��Ӧ������������ѧ����ʽΪ��2Na2O2+2CO2�T2Na2CO3+O2��Ϊ����֤�÷�Ӧ�������IJ�����ij��ȤС���ͬѧ�������ͼ��ʾ��ʵ�飮
��1��ʵ����ȷ�����巢��װ��ʱӦ���ǵ�������
��2����ͼ2��ʵ������ȡCO2��װ�ã�����װ�õ������Եķ����ǣ���װ���е������ϵĽ�Ƥ���õ��ɼм�ס��������©����ע��ˮ��Һ��߳�©�������¶˹ܿڣ����ܹ۲쵽
��3�����A��Bװ�õ�Ŀ���Ǿ�������Ĵ����Ҹ����CO2����Bװ�õľ��幦����
��4��Ϊ��ȷ��CO2��Na2O2��Ӧ���������������������Ҫ���õ���֤ʵ�������
��5������������̼û�б�����������ȫ���յ�������
��6��������ˮҲ����Na2O2��Ӧ�������������������ƣ�д���÷�Ӧ�Ļ�ѧ����ʽ��

��1��ʵ����ȷ�����巢��װ��ʱӦ���ǵ�������
��Ӧ���״̬�ͷ�Ӧ������
����ȡ������̼��װ�ã���ͼ1��ͼ2��ͼ3�У�ѡͼ2
��2����ͼ2��ʵ������ȡCO2��װ�ã�����װ�õ������Եķ����ǣ���װ���е������ϵĽ�Ƥ���õ��ɼм�ס��������©����ע��ˮ��Һ��߳�©�������¶˹ܿڣ����ܹ۲쵽
©�������γ�һ���ȶ���ˮ��
������֤��װ�ò�©������3�����A��Bװ�õ�Ŀ���Ǿ�������Ĵ����Ҹ����CO2����Bװ�õľ��幦����
���������̼
����ƿ��Һ��Ӧ��Ũ����
����4��Ϊ��ȷ��CO2��Na2O2��Ӧ���������������������Ҫ���õ���֤ʵ�������
�������ǵ�ľ�����ڼ���ƿ�Ϸ��ĵ��ܿڸ���
����5������������̼û�б�����������ȫ���յ�������
Cװ�ô��ij���ʯ��ˮ�����
����6��������ˮҲ����Na2O2��Ӧ�������������������ƣ�д���÷�Ӧ�Ļ�ѧ����ʽ��
2Na2O2+2H2O�T4NaOH+O2��
��������֪����Ӧ��״̬ȷ��װ�ã�������ʵ��Ҫ�����ʵ����̣������л����о��������֪ʶ��ͨ��Ķ�����̼��Ҫȥ���Ȼ��⼰ˮ������ʵ���зֱ���ò��������̼��Ӧ��̼�����ƺ�Ũ���ᣬ�������ǣ���������Ĵ��ڵ�ʵ�飮
����⣺��1����Ϊ��Ӧ���ڹ�Һ�����������Դ�Ϊ����Ӧ���״̬�ͷ�Ӧ���� ͼ2
��2��ֻҪװ�ò�©������ˮ�IJ��ϼ���װ���������ѹǿ��ϱ��ﵽһ��ѹǿ��©����Һ�岻���½���
�ʴ�Ϊ��©���¶���һ�ȶ���Һ��
��3��������̼���������壬����Ũ���ᷴӦ����ѡ��Ũ�������������
�ʴ�Ϊ���ڸ��������̼������ˮ������Ũ����
��4�������ǵ�ľ����������Ḵȼ����ѡD���ô�����ľ������
��5��������̼ͨ��ʯ��ˮ��ʹʯ��ˮ����ǣ��Ǽ��������̼�ij��÷�������ѡC�е�ʯ��ˮ�����
��6��������Ŀ�������Ϣ��ȷ����д��ѧʽ���ʴ�Ϊ��2Na2O2+2H2O�T4NaOH+O2��
�ʴ�Ϊ��
��1����Ӧ���״̬�ͷ�Ӧ������ ͼ2
��2��©�������γ�һ���ȶ���ˮ������Һ�治���½�����
��3�����������̼��1�֣� Ũ����
��4���������ǵ�ľ�����ڼ���ƿ�Ϸ��ĵ��ܿڸ�����2�֣�
��5��Cװ�ô��ij���ʯ��ˮ����ǣ�2�֣�
��6��2Na2O2+2H2O�T4NaOH+O2����2�֣�
��2��ֻҪװ�ò�©������ˮ�IJ��ϼ���װ���������ѹǿ��ϱ��ﵽһ��ѹǿ��©����Һ�岻���½���
�ʴ�Ϊ��©���¶���һ�ȶ���Һ��
��3��������̼���������壬����Ũ���ᷴӦ����ѡ��Ũ�������������
�ʴ�Ϊ���ڸ��������̼������ˮ������Ũ����
��4�������ǵ�ľ����������Ḵȼ����ѡD���ô�����ľ������
��5��������̼ͨ��ʯ��ˮ��ʹʯ��ˮ����ǣ��Ǽ��������̼�ij��÷�������ѡC�е�ʯ��ˮ�����
��6��������Ŀ�������Ϣ��ȷ����д��ѧʽ���ʴ�Ϊ��2Na2O2+2H2O�T4NaOH+O2��
�ʴ�Ϊ��
��1����Ӧ���״̬�ͷ�Ӧ������ ͼ2
��2��©�������γ�һ���ȶ���ˮ������Һ�治���½�����
��3�����������̼��1�֣� Ũ����
��4���������ǵ�ľ�����ڼ���ƿ�Ϸ��ĵ��ܿڸ�����2�֣�
��5��Cװ�ô��ij���ʯ��ˮ����ǣ�2�֣�
��6��2Na2O2+2H2O�T4NaOH+O2����2�֣�
�������μ�һЩ��������ʼ����ǽ��������Ӽ�����ʵ����Ĺؼ������������̼�ó���ʯ��ˮ�����������ô�����ľ����

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ

 23��������ߺ�DZˮͧ�п��ù������ƣ�Na2O2����Ϊ����������������ȤС��ͬѧΧ�ƹ������ƽ��е�һϵ��̽����������뵽���У�
23��������ߺ�DZˮͧ�п��ù������ƣ�Na2O2����Ϊ����������������ȤС��ͬѧΧ�ƹ������ƽ��е�һϵ��̽����������뵽���У�
