��Ŀ����
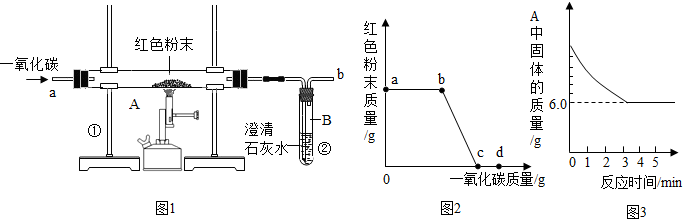
 ������ұ��������������һ����Ҫ��־����ͼ�Ǹ�������ԭ����Ƶ�ʵ��װ��ͼ��
������ұ��������������һ����Ҫ��־����ͼ�Ǹ�������ԭ����Ƶ�ʵ��װ��ͼ����1������д��ұ�������Ļ�ѧ����ʽ��
Fe2O3+3CO
2Fe+3CO2
| ||
Fe2O3+3CO
2Fe+3CO2
��
| ||
��2��Ϊȷ��ʵ�鰲ȫ����ʵ��ǰӦע��������ǣ�
����һ����̼�Ĵ���
����һ����̼�Ĵ���
����3����װ���в����Ƶĵط�����д������һ�ָĽ��ķ�����
�þƾ��ƶ�β����ȼ�������ϴ��ռ�β��
�þƾ��ƶ�β����ȼ�������ϴ��ռ�β��
����������1��һ����̼���������ڸ����������������Ͷ�����̼��д����Ӧ�Ļ�ѧ����ʽ���ɣ�
��2�����ݲ����Ŀ�ȼ�������ڼ���ʱ���ܷ�����ը���з������
��3������β���к�����Ⱦ������һ����̼������һ����̼�����ʽ��з������
��2�����ݲ����Ŀ�ȼ�������ڼ���ʱ���ܷ�����ը���з������
��3������β���к�����Ⱦ������һ����̼������һ����̼�����ʽ��з������
����⣺��1��һ����̼���������ڸ����������������Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪ��Fe2O3+3CO
2Fe+3CO2��
��2��һ����̼���п�ȼ�ԣ�������һ����̼�ڼ���ʱ���ܷ�����ը��Ϊȷ��ʵ�鰲ȫ��ʵ��ǰ����һ����̼�Ĵ��ȣ�
��3��β���к�����Ⱦ������һ����̼������Ҫ����β�����������õ�ȼβ���������ϴ��ռ�β���ķ���������
�ʴ�Ϊ����1��Fe2O3+3CO
2Fe+3CO2��
��2������һ����̼�Ĵ��ȣ�
��3���þƾ��ƶ�β����ȼ�������ϴ��ռ�β����
| ||
��2��һ����̼���п�ȼ�ԣ�������һ����̼�ڼ���ʱ���ܷ�����ը��Ϊȷ��ʵ�鰲ȫ��ʵ��ǰ����һ����̼�Ĵ��ȣ�
��3��β���к�����Ⱦ������һ����̼������Ҫ����β�����������õ�ȼβ���������ϴ��ռ�β���ķ���������
�ʴ�Ϊ����1��Fe2O3+3CO
| ||
��2������һ����̼�Ĵ��ȣ�
��3���þƾ��ƶ�β����ȼ�������ϴ��ռ�β����
�����������ѶȲ�������һ����̼����������ʵ��ԭ����ע�����������ȷ�����Ĺؼ���

��ϰ��ϵ�д�
 �����ҵ��ٿ���������������ϵ�д�
�����ҵ��ٿ���������������ϵ�д�
�����Ŀ


 ��1��������ұ��������������һ����Ҫ��־����ͼ��ʵ����ģ��������װ��ͼ��д��ұ�������Ļ�ѧ����ʽ��
��1��������ұ��������������һ����Ҫ��־����ͼ��ʵ����ģ��������װ��ͼ��д��ұ�������Ļ�ѧ����ʽ��