��Ŀ����
���������Ǵ�����Ⱦ��֮һ��Ϊ���Եزⶨ��Χ�����ж�������ĺ�����ijѧ������С��ļס�������ͬѧ�ֱ�����ͬʵ��װ�ú���Һ���ⶨͬһʱ�䣬ͬһ�ص�����У���SO2��N2��O2���壬����������ԣ�SO2�ĺ�����ʵ��װ����ͼ���Թ���װ�е�ĵ���ϡ��ҺA��
SO2��I2������ӦΪ��N2��O2����I2�����۷�Ӧ����SO2+I2+2H2O=H2SO4+2HI���Իش��������⣺
��1������װ�õ�������ʱ�������Թ���װ��������ˮ����֤�������ܵ��¶˽�û��ˮ�У���Ȼ�� ��
��֤����װ�õ����������ã�
��2����A��Һ������Ϊb g��������������Ϊw������Һ����ɫ�պñ�ɫʱ��I2�պ�ȫ����Ӧ����ֹͣ��������ʱ�ס��������ע��������ȡ����������ֲ�ΪV��L��V��L��SO2�����ڴ�״̬�µ��ܶ�Ϊ��g/L������V����V������õص������SO2�����������ӽ� ���ú�b��w���ѡ�V���ȵĴ���ʽ��ʾ������һ��ʵ���������ϴ�����ԭ������� ��
SO2��I2������ӦΪ��N2��O2����I2�����۷�Ӧ����SO2+I2+2H2O=H2SO4+2HI���Իش��������⣺

��1������װ�õ�������ʱ�������Թ���װ��������ˮ����֤�������ܵ��¶˽�û��ˮ�У���Ȼ��
��֤����װ�õ����������ã�
��2����A��Һ������Ϊb g��������������Ϊw������Һ����ɫ�պñ�ɫʱ��I2�պ�ȫ����Ӧ����ֹͣ��������ʱ�ס��������ע��������ȡ����������ֲ�ΪV��L��V��L��SO2�����ڴ�״̬�µ��ܶ�Ϊ��g/L������V����V������õص������SO2�����������ӽ�
��������1������װ�õ��ص���ѹǿ��֪ʶ������Ū��װ�������Եļ������Ҫ��֤װ�����γ�һ���ܱյ���ϵ�������ܱ���ϵ�ڵ�ѹǿ�ı仯�����ڵ�ע�����ƻ���ʱ������װ���ص㣬�������Թ��ڳ�������ע����������ʱ������װ���ص㣬�������Թ��ڿ���������˼���װ�õ�������ʱ��Ϊʹ�γ�һ���ܱյĿռ䣬Ӧ��ע�����Ļ�����ʹװ���ڵ�ѹǿ��С���Ӷ��ڽ�û��ˮ�еIJ������ܿڻῴ��������ð����
��2������SO2+I2+2H2O=H2SO4+2HI�������ʵ�ǡ����ȫ��Ӧʱ�����������ɵ���ɫ�ͻ���ʧ���Ӷ����ݲμӷ�Ӧ�ĵ�����������������������������Ӷ�ȷ���˵ؿ����ж�������ĺ�����
��2������SO2+I2+2H2O=H2SO4+2HI�������ʵ�ǡ����ȫ��Ӧʱ�����������ɵ���ɫ�ͻ���ʧ���Ӷ����ݲμӷ�Ӧ�ĵ�����������������������������Ӷ�ȷ���˵ؿ����ж�������ĺ�����
����⣺��1����Ҫ�����װ�õ������ԣ��������ⵥ�����ã�����ԭ��˵������ע�����ƻ���ʱ������װ���ص㣬�������Թ��ڳ�������ע����������ʱ������װ���ص㣬�������Թ��ڿ����������Ӧ������ע�����Ļ����������Թ���ѹǿ��С����������û��ˮ�еIJ����ܿڴ�������ð����˵�����������ã��ʴ�Ϊ��������������ע�����Ļ�������û��ˮ�еIJ������ܿ�������ð����
��2��������ȡ�õ�ˮ��һ���ģ��������ʵ���в��뷴Ӧ�Ķ������������һ���࣬Ϊ��ʹ��������������ȫ�����գ���˳�������ʱҪ�����ؽ��У����ݻ�ѧ����ʽ���㣬����뷴Ӧ�Ķ������������ΪX��
SO2 +I2 +2H2O=H2SO4 +2HI
64 254
X bg��w
=
���X=
g=0.252bwg
���뷴Ӧ�Ķ�����������Ϊ
=
L
���飺������SO2���������������Ϊ
L��V��L��100%=
%
���飺������SO2���������������Ϊ
L��V��x100%
=
%
����V����V��������������ʹ��죬��ɿ�����SO2���ˮδ��ַ�Ӧ�������ϴ������������ʵ�ʷ��ϣ��ʴ�Ϊ��
%������������ʹ��죬��ɿ�����SO2���ˮδ��ַ�Ӧ�������ϴ���
��2��������ȡ�õ�ˮ��һ���ģ��������ʵ���в��뷴Ӧ�Ķ������������һ���࣬Ϊ��ʹ��������������ȫ�����գ���˳�������ʱҪ�����ؽ��У����ݻ�ѧ����ʽ���㣬����뷴Ӧ�Ķ������������ΪX��
SO2 +I2 +2H2O=H2SO4 +2HI
64 254
X bg��w
| 64 |
| X |
| 254 |
| bg��w |
���X=
| 32bw |
| 127 |
���뷴Ӧ�Ķ�����������Ϊ
| 0.252bwg |
| ��g/L |
| 0.252bw |
| �� |
���飺������SO2���������������Ϊ
| 0.252bw |
| �� |
| 0.00252bw |
| ��V�� |
���飺������SO2���������������Ϊ
| 0.252bw |
| �� |
=
| 0.00252bw |
| ��V�� |
����V����V��������������ʹ��죬��ɿ�����SO2���ˮδ��ַ�Ӧ�������ϴ������������ʵ�ʷ��ϣ��ʴ�Ϊ��
| 0.00252bw |
| ��V�� |
��������Ҫ�Կ����ж�������ĺ���������̽����������һ������ȣ��ѶȽϴ���Ҫ����ѧ���������⡢��������������

��ϰ��ϵ�д�
 ���ɿ��õ�Ԫ����AB��ϵ�д�
���ɿ��õ�Ԫ����AB��ϵ�д� С�����ϵ�д�
С�����ϵ�д�
�����Ŀ
 ���������Ǵ�����Ⱦ��֮һ���ҹ��Ļ��������������жԿ����ж�����������Ũ�ȣ���λ����Ŀ��������������������������ֵ�����ʾ��
���������Ǵ�����Ⱦ��֮һ���ҹ��Ļ��������������жԿ����ж�����������Ũ�ȣ���λ����Ŀ��������������������������ֵ�����ʾ��| Ũ����ֵ��mg/m3�� |
| һ���� ������ ������ 0.15 0.50 0.70 |
 ���������Ǵ�����Ⱦ��֮һ���ҹ��Ŀ����������жԿ����ж�����������Ũ�ȣ���λ����Ŀ��������������������������ֵ���±���ʾ��
���������Ǵ�����Ⱦ��֮һ���ҹ��Ŀ����������жԿ����ж�����������Ũ�ȣ���λ����Ŀ��������������������������ֵ���±���ʾ��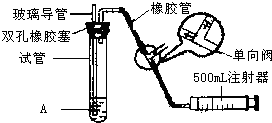 ���������Ǵ�����Ⱦ��֮һ��Ϊ���Եزⶨ��Χ�����е�SO2������ijѧ������С���������ͼ��ʵ��װ�ã�
���������Ǵ�����Ⱦ��֮һ��Ϊ���Եزⶨ��Χ�����е�SO2������ijѧ������С���������ͼ��ʵ��װ�ã�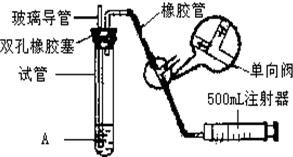 ���������Ǵ�����Ⱦ��֮һ��Ϊ���Եزⶨ��Χ�����е�SO2������ijѧ������С���������ͼ��ʵ��װ�ã�
���������Ǵ�����Ⱦ��֮һ��Ϊ���Եزⶨ��Χ�����е�SO2������ijѧ������С���������ͼ��ʵ��װ�ã�