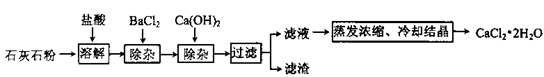
��Ŀ����
��2011��㶫���ݣ�27��)�Ӻ�ˮ�п�����ȡþ�����е�һ�������ǽ�±ˮ(��Mgcl2��NaCl��KCl�Ļ�� Һ)ת��Ϊ�Ȼ�þ���塣ʵ������ģ���ת���Ĺ������£��ش��й����⣺
(1)�Ʊ�������þ����±ˮ�м���������ʯ��(CaO)����ַ�Ӧ����ˡ�
�ٸù����з����˶����Ӧ�����л�������ȵķ�Ӧ��(�ѧ����ʽ)_________________
�ڹ��˲������õ��IJ����������ձ��⣬����__________________________��
(2)�Ƹ��Ȼ�þ��Һ���������ܽ�Mg(OH)2��Ϊ�˱��ں�������������˹���
�ٷ�Ӧ�Ļ�ѧ����ʽ��_____________________________________��
��ͨ�����㣬��ȷ֪����������������������ܽ�5.8gMg(OH)2��Ҫ36. 5��HCl��Һ
������ȷ����Mg(OH)2�IJ��跱����ʵ���в������ã�����ͨ��һ�������������ﵽ
����������������Ŀ�ġ������ǣ���Mg(OH)2�����ձ��У�___________________________
ֱ��Mg(OH)2��ȫ�ܽ�Ϊֹ��
(3)�Ƹ��Ȼ�þ���壺����Ũ��MgCl2��Һ����ȴ�ᾧ
(1)�Ʊ�������þ����±ˮ�м���������ʯ��(CaO)����ַ�Ӧ����ˡ�
�ٸù����з����˶����Ӧ�����л�������ȵķ�Ӧ��(�ѧ����ʽ)_________________
�ڹ��˲������õ��IJ����������ձ��⣬����__________________________��
(2)�Ƹ��Ȼ�þ��Һ���������ܽ�Mg(OH)2��Ϊ�˱��ں�������������˹���
�ٷ�Ӧ�Ļ�ѧ����ʽ��_____________________________________��
��ͨ�����㣬��ȷ֪����������������������ܽ�5.8gMg(OH)2��Ҫ36. 5��HCl��Һ
������ȷ����Mg(OH)2�IJ��跱����ʵ���в������ã�����ͨ��һ�������������ﵽ
����������������Ŀ�ġ������ǣ���Mg(OH)2�����ձ��У�___________________________
ֱ��Mg(OH)2��ȫ�ܽ�Ϊֹ��
(3)�Ƹ��Ȼ�þ���壺����Ũ��MgCl2��Һ����ȴ�ᾧ
�Ţ�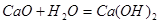 ��©����������
��©����������
�Ƣ�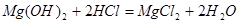
��20 ���ý�ͷ�ι���μ���ϡ������Һ
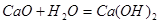 ��©����������
��©����������
�Ƣ�
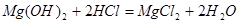
��20 ���ý�ͷ�ι���μ���ϡ������Һ
��������1���������ܺ�ˮ��Ӧ�����������ƣ�ͬʱ�ų��������ȣ����˲������õ��IJ����������ձ�����������©����
��2�������������þ��Ӧ�������Ȼ�þ��ˮ�����ݻ�ѧ����ʽ���Խ�����ط���ļ��㣻���Կ���ϡ������������Ӷ��ﵽǡ����ȫ��Ӧ��Ŀ�ģ�
��𣺽⣺��1���������ƺ�ˮ��Ӧ�ܹ��ų��������ȣ���Ӧ�Ļ�ѧ����ʽΪ��CaO+H2O=Ca��OH��2��
�ڹ��˲������õ��IJ����������ձ��⣬����©���������������©������������
��2���������������þ��Ӧ�Ļ�ѧ����ʽ�ǣ�Mg��OH��2+2HCl=MgCl2+2H2O��
�ڽ����ܽ�5.8gMg��OH��2��Ҫ�Ȼ��������ΪX��
Mg��OH��2+2HCl=MgCl2+2H2O
58 73
5.8g X
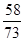 =
= X=7.3g
X=7.3g
��Ҫ36.5%HCl��Һ������Ϊ��7.3g��36.5%=20g��
����Ҫ36.5%HCl��Һ������Ϊ20g��
�۲����ǣ���Mg��OH��2�����ձ��У��ý�ͷ�ι���μ���ϡ������Һֱ��Mg��OH��2��ȫ�ܽ�Ϊֹ������ý�ͷ�ι���μ���ϡ������Һ��
��2�������������þ��Ӧ�������Ȼ�þ��ˮ�����ݻ�ѧ����ʽ���Խ�����ط���ļ��㣻���Կ���ϡ������������Ӷ��ﵽǡ����ȫ��Ӧ��Ŀ�ģ�
��𣺽⣺��1���������ƺ�ˮ��Ӧ�ܹ��ų��������ȣ���Ӧ�Ļ�ѧ����ʽΪ��CaO+H2O=Ca��OH��2��
�ڹ��˲������õ��IJ����������ձ��⣬����©���������������©������������
��2���������������þ��Ӧ�Ļ�ѧ����ʽ�ǣ�Mg��OH��2+2HCl=MgCl2+2H2O��
�ڽ����ܽ�5.8gMg��OH��2��Ҫ�Ȼ��������ΪX��
Mg��OH��2+2HCl=MgCl2+2H2O
58 73
5.8g X
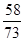 =
= X=7.3g
X=7.3g��Ҫ36.5%HCl��Һ������Ϊ��7.3g��36.5%=20g��
����Ҫ36.5%HCl��Һ������Ϊ20g��
�۲����ǣ���Mg��OH��2�����ձ��У��ý�ͷ�ι���μ���ϡ������Һֱ��Mg��OH��2��ȫ�ܽ�Ϊֹ������ý�ͷ�ι���μ���ϡ������Һ��

��ϰ��ϵ�д�
�����Ŀ
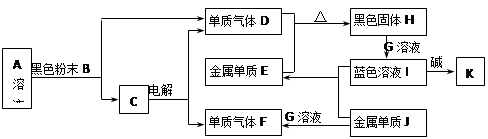
 C + D���� ����
C + D���� ����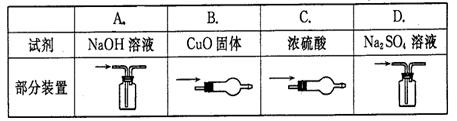


 �����������¡�ԭ��ʯ��ʯ�к���������Ҫ��
�����������¡�ԭ��ʯ��ʯ�к���������Ҫ��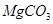 ��
��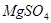 ��
��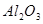 ��
��