��Ŀ����
ͨ������Ļ�ѧѧϰ��������ʶ�ˡ��ᡱ�͡������ش��������⣺
��1���������������ᡢ����ȣ������ǵ�ˮ��Һ�����ڴ�������ͬ�� ���ӣ�����ţ�����ˣ������кܶ����ƵĻ�ѧ���ʣ��磺 �� ���ȣ�д�������ɣ���
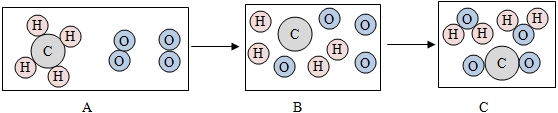
��2�������������ơ��������Ƶȣ��������ƿ���ijЩ����ĸ���������������Ʋ����������������̼��ԭ����[д����ѧ����ʽ] ��
�����壬�������ƿ�����ʯ����ˮ��Ӧ�Ƶã���ѧ����ʽΪ�� ��
��3��5?12�봨������ֺ�������Ϊ����������Ⱥ�ڵ�����ˮ��ȫ���������߲���Ҫ����������ˮԴ���м�⣬��ÿɿ��Ŀ�ѧ���ݣ�������һƿˮ����Ҫ��õ��������ȣ�Ӧ��β����� ��
��1���������������ᡢ����ȣ������ǵ�ˮ��Һ�����ڴ�������ͬ��
��2�������������ơ��������Ƶȣ��������ƿ���ijЩ����ĸ���������������Ʋ����������������̼��ԭ����[д����ѧ����ʽ]
�����壬�������ƿ�����ʯ����ˮ��Ӧ�Ƶã���ѧ����ʽΪ��
��3��5?12�봨������ֺ�������Ϊ����������Ⱥ�ڵ�����ˮ��ȫ���������߲���Ҫ����������ˮԴ���м�⣬��ÿɿ��Ŀ�ѧ���ݣ�������һƿˮ����Ҫ��õ��������ȣ�Ӧ��β�����
���㣺��Ļ�ѧ����,��Һ�����Ȳⶨ,��ʯ�ҵ���������;,��Ļ�ѧ����,��д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ
ר�⣺�������� ���ͨ��,�����ļ� ���ͨ��
��������1��������Ķ��壺������ˮ��Һ�е������������ȫ���������ӵĻ����������ͨ�ԣ�
��2�������������ܹ���Ӧ�����Լ������������������ȣ���ʯ��������ʯ�Һ�ˮ��Ӧ�õ���
��3���ⶨ��Һ������������Dz���PH��ֽ���м��飮
��2�������������ܹ���Ӧ�����Լ������������������ȣ���ʯ��������ʯ�Һ�ˮ��Ӧ�õ���
��3���ⶨ��Һ������������Dz���PH��ֽ���м��飮
����⣺��1����Ϊ������ˮ��Һ�е������������ȫ���������ӵĻ������������ˮ��Һ�о�����ͬ��H+��
�������ͨ�Կ�֪����ʹ��ɫʯ����Һ��죬ʹ��ɫ��̪��Һ����ɫ���������кͷ�Ӧ������ϻ��ý�����Ӧ���ܺͽ��������ﷴӦ���ܺͲ����η�Ӧ�ȣ�
��2���������Ƶ����ڼ���������������Ե������������̼�Ͷ�������ȣ���Ϊ������֮��Ӧ��2NaOH+CO2=Na2CO3+H2O��
��ʯ��������ʯ�Һ�ˮ��Ӧ�õ����䷴Ӧ����ʽΪ��CaO+H2O=Ca��OH��2��
��3��ȡ��һ��pH��ֽ���ò�������ͷ�ι�ȡ��������Һ��������ֽ�ϣ���һ�������ɫ���Աȣ�Ȼ�����pH��ֵ��
�ʴ�Ϊ����1��H+���������Ӧ������������ﷴӦ��
��2��2NaOH+CO2=Na2CO3+H2O��CaO+H2O=Ca��OH��2��
��3��ȡ��һ��pH��ֽ���ò�������ͷ�ι�ȡ��������Һ��������ֽ�ϣ���һ�������ɫ���Աȣ�Ȼ�����pH��ֵ��
�������ͨ�Կ�֪����ʹ��ɫʯ����Һ��죬ʹ��ɫ��̪��Һ����ɫ���������кͷ�Ӧ������ϻ��ý�����Ӧ���ܺͽ��������ﷴӦ���ܺͲ����η�Ӧ�ȣ�
��2���������Ƶ����ڼ���������������Ե������������̼�Ͷ�������ȣ���Ϊ������֮��Ӧ��2NaOH+CO2=Na2CO3+H2O��
��ʯ��������ʯ�Һ�ˮ��Ӧ�õ����䷴Ӧ����ʽΪ��CaO+H2O=Ca��OH��2��
��3��ȡ��һ��pH��ֽ���ò�������ͷ�ι�ȡ��������Һ��������ֽ�ϣ���һ�������ɫ���Աȣ�Ȼ�����pH��ֵ��
�ʴ�Ϊ����1��H+���������Ӧ������������ﷴӦ��
��2��2NaOH+CO2=Na2CO3+H2O��CaO+H2O=Ca��OH��2��
��3��ȡ��һ��pH��ֽ���ò�������ͷ�ι�ȡ��������Һ��������ֽ�ϣ���һ�������ɫ���Աȣ�Ȼ�����pH��ֵ��
���������⿼�����ͨ�ԣ�����ĸ�����ӣ��ᡢ��ε��ܽ��ԣ�Ҫ��ѧ��Ҫ������ͨ�ԡ��ᡢ��ε��ܽ��ԣ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
���л�ѧ����ɱ�ʾһ��Ԫ�أ�Ҳ�ɱ�ʾ��Ԫ�ص�һ��ԭ�ӣ����ɱ�ʾ��Ԫ�ص�һ�����ʵ��ǣ�������
| A��H | B��Cl | C��Fe | D��CO |