��Ŀ����
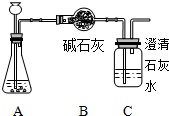
ij��ѧѧϰС����������ϡ�����15gʯ��ʯ��Ʒ�����ʲ�����ˮ�Ҳ����ᷴӦ��������ϡ����Ļӷ������п�ѧ̽����ʵ��װ�����£�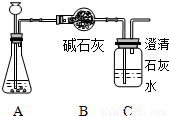
�й��������£�
��Aװ�������������ʱ���Bװ�õ�����������4.7g��Cװ�õ�����û�����仯������������
����ش�
��1��ʯ��ʯ��Ʒ��̼��Ƶ�����Ϊ______g��
��2��15g��Ʒ��������ɶ�����̼���������Ϊ______g��
��3������ϡ������������������Ƕ��٣�д��������̣�
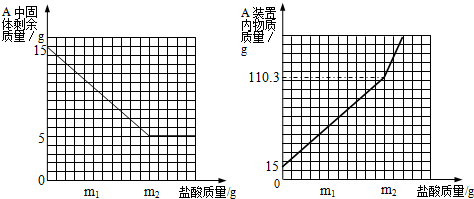
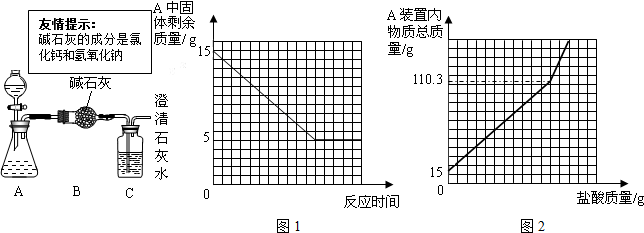
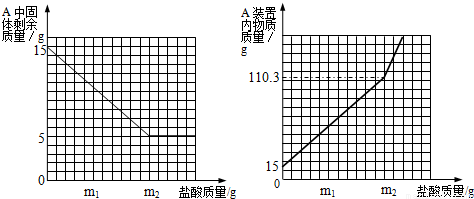
���𰸡���������1��ͨ���۲�A��ʣ����������ͼ�������Ĺ�ϵ��֪���������������������m2gʱ��ʣ�������������ڼ��٣��������֪ʣ����������ʣ����ʵ�������5g�����֪ʯ��ʯ��Ʒ��̼��Ƶ�������
��2����̼��Ƶ�����������̼��ƺ����ᷴӦ�ķ���ʽ����ʾ��������ϵ���Ϳ������15g��Ʒ��������ɶ�����̼���������������������μӷ�Ӧ��ϡ���������ʵ�������
��3�����������غ㶨�ɣ��ɷ�Ӧ����Һ���������μӷ�Ӧ��̼��Ƶ����������ɵĶ�����̼����������������μӷ�Ӧ��������Һ���������ڸ������ʵ����������ļ��㹫ʽ�Ϳ��������ϡ�������������������
����⣺��1����A��ʣ����������ͼ�������Ĺ�ϵ��֪�����ʵ�������5g��ʯ��ʯ��Ʒ��̼��Ƶ�������15g-5g=10g��
��2����15g��Ʒ�е�̼�����ȫ�����ᷴӦʱ���ɵĶ�����̼��࣬
�����ɶ�����̼������Ϊx���μӷ�Ӧ�����������ʵ�����Ϊy
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 73 44
10g y x
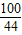 =
= x=4.4g
x=4.4g
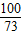 =
=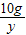 y=7.3g
y=7.3g
��3���������⣬A�����µ�����110.3��Ӧ�����������������ʯ��ʯ��Ʒ��������ȥA�лӷ��ߵ�ˮ�Ͷ�����̼����������B���ص�����4.7�ˣ�������ʽΪ��m2+15g-4.7g=110.3g��m2=100g��
����ϡ������������������� ×100%=7.3%��
×100%=7.3%��
�𣺣�1��ʯ��ʯ��Ʒ��̼��Ƶ�����Ϊ10 g����2��15g��Ʒ��������ɶ�����̼���������Ϊ 4.4g����3������ϡ�������������������7.3%��
������������Ҫ�������й��������������뻯ѧ����ʽ���ۺϼ��㣬������ѵ��Ƿ�Ӧǰ���й���Һ�����ķ����ж����ж������ݾ��������غ㶨�ɣ�
��2����̼��Ƶ�����������̼��ƺ����ᷴӦ�ķ���ʽ����ʾ��������ϵ���Ϳ������15g��Ʒ��������ɶ�����̼���������������������μӷ�Ӧ��ϡ���������ʵ�������
��3�����������غ㶨�ɣ��ɷ�Ӧ����Һ���������μӷ�Ӧ��̼��Ƶ����������ɵĶ�����̼����������������μӷ�Ӧ��������Һ���������ڸ������ʵ����������ļ��㹫ʽ�Ϳ��������ϡ�������������������
����⣺��1����A��ʣ����������ͼ�������Ĺ�ϵ��֪�����ʵ�������5g��ʯ��ʯ��Ʒ��̼��Ƶ�������15g-5g=10g��
��2����15g��Ʒ�е�̼�����ȫ�����ᷴӦʱ���ɵĶ�����̼��࣬
�����ɶ�����̼������Ϊx���μӷ�Ӧ�����������ʵ�����Ϊy
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 73 44
10g y x
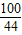 =
= x=4.4g
x=4.4g 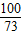 =
=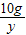 y=7.3g
y=7.3g��3���������⣬A�����µ�����110.3��Ӧ�����������������ʯ��ʯ��Ʒ��������ȥA�лӷ��ߵ�ˮ�Ͷ�����̼����������B���ص�����4.7�ˣ�������ʽΪ��m2+15g-4.7g=110.3g��m2=100g��
����ϡ�������������������
 ×100%=7.3%��
×100%=7.3%���𣺣�1��ʯ��ʯ��Ʒ��̼��Ƶ�����Ϊ10 g����2��15g��Ʒ��������ɶ�����̼���������Ϊ 4.4g����3������ϡ�������������������7.3%��
������������Ҫ�������й��������������뻯ѧ����ʽ���ۺϼ��㣬������ѵ��Ƿ�Ӧǰ���й���Һ�����ķ����ж����ж������ݾ��������غ㶨�ɣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ