��Ŀ����
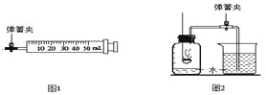
����Ŀ��ijʵ��С����28 g����أ�KClO3����������̵Ļ���ﹲ����ȡ��������Ӧ�Ļ�ѧ����ʽΪ��2KClO3![]() 2KCl + 3O2�������������������ʱ����ʣ���������Ϊ18.4 g��ʵ���ʣ�����������ˮ����ܽ⣬���ˣ����ն������̹��壬���õ��Ȼ�����Һ100 g��
2KCl + 3O2�������������������ʱ����ʣ���������Ϊ18.4 g��ʵ���ʣ�����������ˮ����ܽ⣬���ˣ����ն������̹��壬���õ��Ȼ�����Һ100 g��
����㣺
��1����ȡ����������Ϊ g��
��2��������������������
��3���Ȼ�����Һ�����ʵ�����������
���𰸡���1��9.6 ��2��3.5g ��3��14.9%
��������
�����������1�����������غ㶨�ɣ���ѧ��Ӧǰ�����ʵ����������䣬����ȡ����������=28g -18.4 g =9.6g��2�����ݻ�ѧ����ʽ��KClO 3![]() 2KCl��3O2����O2��KClO3��KCl��������ϵ���ɷֱ����KClO3��KCl����������һ������Ȼ�����Һ�����ʵ���������
2KCl��3O2����O2��KClO3��KCl��������ϵ���ɷֱ����KClO3��KCl����������һ������Ȼ�����Һ�����ʵ���������
�⣺������ص�����Ϊx�������Ȼ��ص�����Ϊy
2KClO3![]() 2KCl��3O2��
2KCl��3O2��
245 149 96
x y 9.6 g
245:96=x:9.6g x=24.5g
��2��������ж������̵�����Ϊ��28 g��24.5 g = 3.5 g
149:96=x:9.6g y=14.9g
��3���Ȼ�����Һ�����ʵ���������= 14.9 g/100 g��100%=14.9%

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�