��Ŀ����
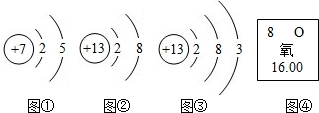
ͼ�١������������Ľṹʾ��ͼ��ͼ������Ԫ�������ڱ��е������Ϣ��
������ѧ֪ʶ�ش�
��1��ͼ�ٱ�ʾ����
��2��ͼ�ں�ͼ�۱�ʾ����
��3����ͼ��������Ϣ��֪��Ԫ�ص�ԭ�Ӻ����������

������ѧ֪ʶ�ش�
��1��ͼ�ٱ�ʾ����
�ǽ���
�ǽ���
Ԫ�أ�������������ǽ�������ϡ�����塱������Ԫ���γɵĵ����ڿ�������ռ�����������Լ��78%
78%
����2��ͼ�ں�ͼ�۱�ʾ����
ͬ��
ͬ��
���ͬ�֡���ͬ�֡���Ԫ�أ�����ͼ�ڱ�ʾ����������Al3+
Al3+
����Ԫ�ط��ű�ʾ������3����ͼ��������Ϣ��֪��Ԫ�ص�ԭ�Ӻ����������
8
8
����Ԫ����ͼ�۱�ʾ��Ԫ���γɻ�����Ļ�ѧʽ��Al2O3
Al2O3
����������1������ͼ�ٵ�Ԫ�������Լ��γɵĵ������������ɣ�
��2������ͬ��Ԫ����������ͬ���Լ�ͼ�������Ĵ���������ɣ�
��3��������Ԫ�ص��������Լ������Ļ��ϼ۷������
��2������ͬ��Ԫ����������ͬ���Լ�ͼ�������Ĵ���������ɣ�
��3��������Ԫ�ص��������Լ������Ļ��ϼ۷������
����⣺��1��ͼ1��ʾ��Ԫ���ǵ�Ԫ�أ����ڷǽ���Ԫ�أ��䵥���ǵ������ڿ����е����������78%��
��2��ͬ��Ԫ����������ͬ������ͼ�ں�ͼ�۱�ʾ�Ķ�����Ԫ�أ�ͼ2�����������Ⱥ����������3������ͼ�ڱ�ʾ����������Al3+��
��3����ͼ��������Ϣ��֪��Ԫ�ص�ԭ������Ϊ8��������������Ϊ8������������ҲΪ8����Ԫ�صĻ��ϼ۳���-2�ۣ���Ԫ�س���+3�ۣ�������Ԫ����ͼ�۱�ʾ��Ԫ���γɻ�����Ļ�ѧʽ��Al2O3��
�ʴ�Ϊ����1���ǽ�����78%����2��ͬ�֣�Al3+����3��8��Al2O3��
��2��ͬ��Ԫ����������ͬ������ͼ�ں�ͼ�۱�ʾ�Ķ�����Ԫ�أ�ͼ2�����������Ⱥ����������3������ͼ�ڱ�ʾ����������Al3+��
��3����ͼ��������Ϣ��֪��Ԫ�ص�ԭ������Ϊ8��������������Ϊ8������������ҲΪ8����Ԫ�صĻ��ϼ۳���-2�ۣ���Ԫ�س���+3�ۣ�������Ԫ����ͼ�۱�ʾ��Ԫ���γɻ�����Ļ�ѧʽ��Al2O3��
�ʴ�Ϊ����1���ǽ�����78%����2��ͬ�֣�Al3+����3��8��Al2O3��
������������Ҫ�������ӵĽṹʾ��ͼ����ʾ������Ϣ�����壬�ѶȲ������ͻ�����

��ϰ��ϵ�д�
 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д�
�����Ŀ
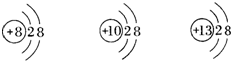 17����ͼ���������Ľṹʾ��ͼ���й����ǵ�˵����ȷ���ǣ�������
17����ͼ���������Ľṹʾ��ͼ���й����ǵ�˵����ȷ���ǣ�������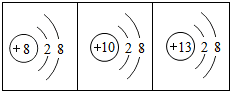 ��ͼ���������Ľṹʾ��ͼ���й����ǵ�˵��������ǣ�������
��ͼ���������Ľṹʾ��ͼ���й����ǵ�˵��������ǣ�������
 ��ͼ���������Ľṹʾ��ͼ���й����ǵ�˵����ȷ���ǣ�������
��ͼ���������Ľṹʾ��ͼ���й����ǵ�˵����ȷ���ǣ�������