��Ŀ����
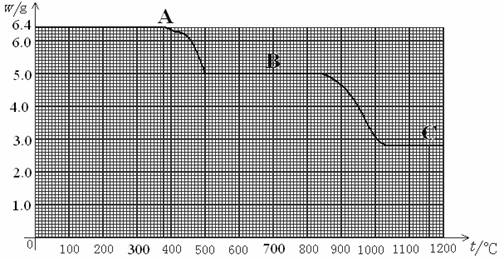
��ˮ�еĸ�����ת��Ϊ����ƣ�CaC2O4������з�Ӧ������DzⶨˮӲ�ȵ�һ�ַ�������ͼ��6.4g����ƹ��������ȷֽ���������ù��������������¶ȱ仯�����ߣ�ͼ��A��B��C�ֱ�������ֹ��壮������ͼ����Ϣ�����ѧ��֪ʶ���ش��������⣺
��1��6.4g�����A��ȫ�ֽ�Ϊ̼���B��COʱ�����ɵ�CO��������______g��
��2�����¶ȴ�500������840��ʱ��Ϊʲô̼��Ƶ��������ֲ���______��
��3���Ծ�ͼ���㲢�ƶ�C�ĺ�����ѧʽ��

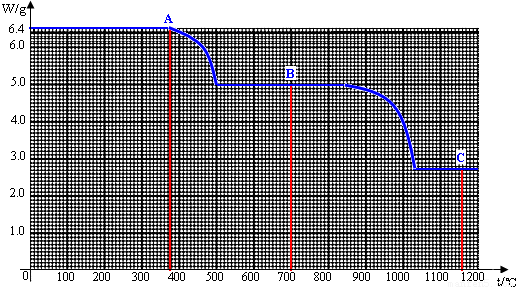
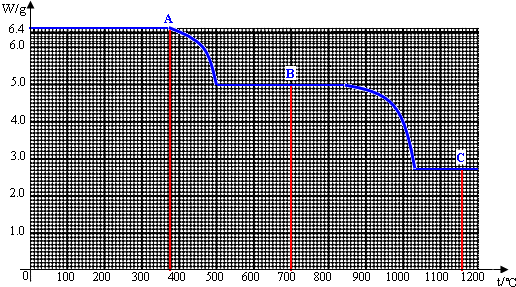
��1��6.4g�����A��ȫ�ֽ�Ϊ̼���B��COʱ�����ɵ�CO��������______g��
��2�����¶ȴ�500������840��ʱ��Ϊʲô̼��Ƶ��������ֲ���______��
��3���Ծ�ͼ���㲢�ƶ�C�ĺ�����ѧʽ��
��1��������CO������Ϊx��CaCO3������Ϊy������Ʒֽ�Ϊ̼��ƺ�CO�ķ���ʽΪ��
CaC2O4
CaCO3+CO
128 100 28
6.4 y x
��
=
=
��֮�ã�x=1.4g y=5g
������CO������Ϊ1.4g��
��2����Ϊ̼���ֻ���ڸ����²ŷֽ⣬����̼��Ƶ��������ֲ��䣮
��3������ͼ���Կ�������C������Ϊ2.8g������Ϊ̼����ڸ���ʱ�ɷֽ�ΪCaO��CO2�����Բ²�C�������ƣ�CaO������֤���£�
CaCO3
CaO+CO2
100 56
5 2.8
�����C�������ƣ���ѧʽCaO��
CaC2O4
| ||
128 100 28
6.4 y x
��
| 128 |
| 6.4 |
| 100 |
| y |
| 28 |
| x |
��֮�ã�x=1.4g y=5g
������CO������Ϊ1.4g��
��2����Ϊ̼���ֻ���ڸ����²ŷֽ⣬����̼��Ƶ��������ֲ��䣮
��3������ͼ���Կ�������C������Ϊ2.8g������Ϊ̼����ڸ���ʱ�ɷֽ�ΪCaO��CO2�����Բ²�C�������ƣ�CaO������֤���£�
CaCO3
| ||
100 56
5 2.8
�����C�������ƣ���ѧʽCaO��

��ϰ��ϵ�д�
�����Ŀ
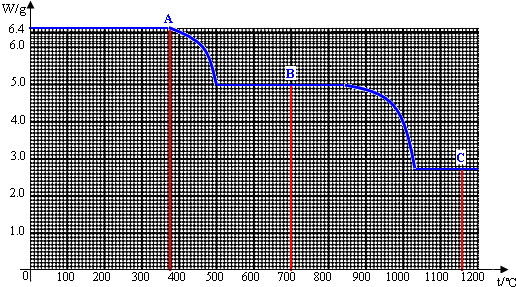
 ��2012?��̳��һģ����ˮ�еĸ�����ת��Ϊ����ƣ�CaC2O4������з�Ӧ������DzⶨˮӲ�ȵ�һ�ַ�������֪�������400�桫500��ʱ������Ӧ�Ļ�ѧ����ʽ��CaC2O4
��2012?��̳��һģ����ˮ�еĸ�����ת��Ϊ����ƣ�CaC2O4������з�Ӧ������DzⶨˮӲ�ȵ�һ�ַ�������֪�������400�桫500��ʱ������Ӧ�Ļ�ѧ����ʽ��CaC2O4