��Ŀ����
��ʡij���п���ѧʵ������������ĸ����⣺������һ������������������Һ���ڼ�Ļ�ѧ���ʣ���CO2����ȡ���ռ�����������O2����ȡ���ռ������������Եķ������ɿ�����ǩȷ�����⣬С��ͬѧ��ǩ���ʦ��������������������ҩƷ��ʵ��̨ǰ��
��ش�
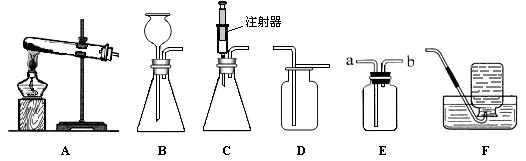
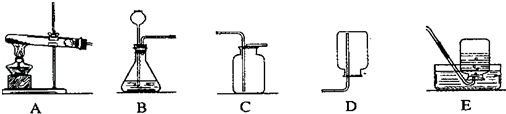
��1��ָ��ͼ1�б�����������ƣ�a b ��
��2����ʵ��̨���ṩ��������ҩƷ������ΪС��鵽���ǵ� �����⣻
��3��ͼ2��С����ɸ�ʵ����Ҫ�������̵�ʾ��ͼ�������ֱ���ÿ�������ȷ��1�֣��ܹ�5�֣�ʵ����Ϻ�С�����3�֣����ҳ�С��ʧ�ֵIJ�����˵��ԭ�� �� ��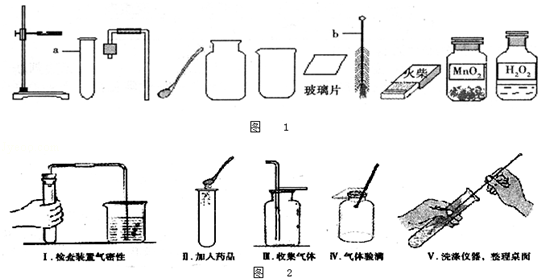
��4����������������ҩƷ��ѡ����Ҳ�������һ�ֳ��������ʵ����ȡ����ѧ����ʽΪ �������� ����һ�ֲ����������ƣ�������װ���������ȡ�����ķ���װ�ã�
��1��a���Թ�b���Թ�ˢ��2���ܣ�3������ֱ���Թܿڼ���ҩƷ��ҩƷ��մ�ڹܿڻ�ܱڣ��ô�����ľ������ƿ��������������֤�Ƿ��ռ�����4��CaCO3+2HCl=CaCl2+CO2��+H2O���ƾ���
���������������1���Թ��dz��õķ�Ӧ�������Թ�ˢ��ˢ�Թܵ��������ʴ�Ϊ��a���Թ�b���Թ�ˢ��2����ʵ��̨���ṩ��������ҩƷ������ΪС��鵽����O2����ȡ���ռ����������ʴ�Ϊ���ܣ�3��С����ɸ�ʵ����Ҫ���������д�����У�����ֱ���Թܿڼ���ҩƷ��ҩƷ��մ�ڹܿڻ�ܱڣ��ô�����ľ������ƿ��������������֤�Ƿ��ռ������ʴ�Ϊ������ֱ���Թܿڼ���ҩƷ��ҩƷ��մ�ڹܿڻ�ܱڣ��ô�����ľ������ƿ��������������֤�Ƿ��ռ�����4��̼��ƺ����ụ�ཻ���ɷ������Ȼ��ƺ�ˮ�Ͷ�����̼����ƽ���ɣ������Ӿƾ��ƣ�������װ���������ȡ�����ķ���װ�ã��ʴ�Ϊ��CaCO3+2HCl=CaCl2+CO2��+H2O���ƾ���
���㣺��������ķ���װ�ú��ռ�װ����ѡȡ������ʵ������ȡ�����ķ�Ӧԭ���������ļ������������д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ��

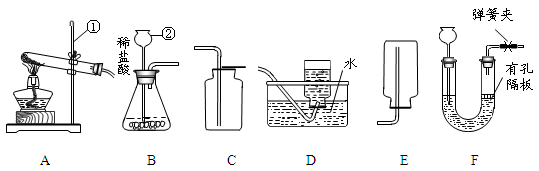
��ͼ��ʵ���ҳ��õ�װ�ã������ͼ�ش��������⣺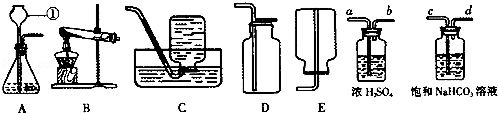
��1��д�����б�����������ƣ����� ��
��2��ʵ�����ø��������ȡ�����Ļ�ѧ����ʽ���� ������ѡ�õķ���װ������ ��������ţ���ʵ����Ϻ�Ҫ�Ƚ����ܴ�ˮ����ȡ����Ϩ��ƾ��ƣ���ԭ������
��3���ռ�ij����ֻ�ܲ���Eװ�ã��ɴ��Ʋ��������е��������� ��
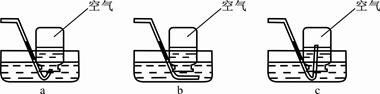
��4��ʵ�����Ƶõ�CO2�����к����Ȼ����ˮ������Ϊ�˵õ������������CO2���壬����װ�õĵ���������������˳������ ������ѡ�����ţ�
| A��a��b��c��d�� | B��b��a��c��d�� | C��c��d��a��b�� | D��d��c��b��a |
ʵ���ҳ��ü�������غͶ������̻����ķ�����ȡ��������ش��������⣺
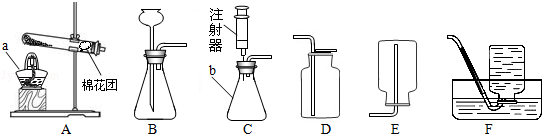
A B C D E
�ŷ�Ӧ�Ļ�ѧ����ʽΪ ��
�����������������a________________ b_______________��
��������������װ��������Ӧ��������װ�ã���ѡ��ķ���װ��Ϊ________��Bװ���Թܿ�Ҫ��
������б��ԭ����_______________________________________________���ռ�װ��Ϊ__________��
����װ��ѡ��������� ������ĸ����
| A�����ڹ̹��ͼ��ȵķ�Ӧ | B�����ڹ�Һ�Ͳ����ȵķ�Ӧ |
| C����ȡ�������ܶȱȿ����� | D����ȡ������������ˮ |

|
| ʵ����� | ��������������ص������� | ��ʱ���룩 |
| 1 | 6��6 | 42 |
| 2 | 2.5:5 | 25 |
| 3 | 2:5 | 34 |
| 4 | 3:10 | 65 |
| 5 | 2:10 | 41 |
��ʵ�����2��3���ݿ��Կ�����ʵ����� ����2��3����Ӧ�ٶ���졣ͨ��������֪��
�ڻ�ѧ��Ӧ�д�������������ǡ����ǡ��� Խ��Խ�á�
�����ڷ�Ӧ��IJ��������ᴿKCl,��������²�������ѿ�ȱ�����ϣ�
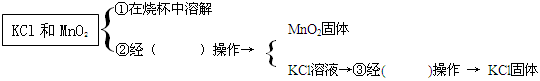
�ڢ١��ڡ��۵IJ����о��õ���������___________��