��Ŀ����
(7��)ˮ���ճ������ũҵ��������ѧ�о�����������Ҫ���㷺����;��
(1)�ӻ�ѧ�ӽǿ�ˮ������ɽǶȿ������ˮʵ��֤����ˮ��____��ɣ������Ƕȿ���ˮ��____(����ӡ�����ԭ�ӡ������ӡ�)���ɡ�
(2)ˮ�ܲμ����ѧ��Ӧ����ͼ��һ�ּ��û���������Һ��������ԭ���ǵ�ⱥ���Ȼ�����Һ���Ƶõ�����Һ�н�ǿ��ɱ���������÷�Ӧ�������Ȼ��ƺ�ˮ��ͨ�������������������ơ��������������÷�Ӧ�Ļ�ѧ����ʽΪ____���������仯�Ƕȿ����÷�Ӧ��____ת��Ϊ��ѧ�ܡ�

(3)ˮ��һ����Ҫ���ܼ����±���ʾ���Dz�ͬ�¶���4������ˮ�е��ܽ�ȡ�
�ش����⣺
������4�����У�30��ʱ�ܽ��������____��
��10��ʱ��16.8gNH4HCO3�������100gˮ�г���ܽ⣬������ҺΪ____ (����͡������͡�)��Һ��
����20��ʱ����NH4HCO3 21 g�ı�����Һ�м�������ʳ�Ρ�����ʳ�εļ��룬�������ľ�����____��
A.NH4HCO3 B. NH4Cl C.NaHCO3
(1)�ӻ�ѧ�ӽǿ�ˮ������ɽǶȿ������ˮʵ��֤����ˮ��____��ɣ������Ƕȿ���ˮ��____(����ӡ�����ԭ�ӡ������ӡ�)���ɡ�
(2)ˮ�ܲμ����ѧ��Ӧ����ͼ��һ�ּ��û���������Һ��������ԭ���ǵ�ⱥ���Ȼ�����Һ���Ƶõ�����Һ�н�ǿ��ɱ���������÷�Ӧ�������Ȼ��ƺ�ˮ��ͨ�������������������ơ��������������÷�Ӧ�Ļ�ѧ����ʽΪ____���������仯�Ƕȿ����÷�Ӧ��____ת��Ϊ��ѧ�ܡ�

(3)ˮ��һ����Ҫ���ܼ����±���ʾ���Dz�ͬ�¶���4������ˮ�е��ܽ�ȡ�
| | 10�� | 20�� | 30�� |
| NaCI | 35.8 | 36.0 | 36.3 |
| NH4HCO3 | 15.8 | 21.0 | 27.O |
| NaHCO3 | 8.1 | 9.6 | 11.1 |
| NH4Cl | 33.3 | 37.2 | 41.4 |
������4�����У�30��ʱ�ܽ��������____��
��10��ʱ��16.8gNH4HCO3�������100gˮ�г���ܽ⣬������ҺΪ____ (����͡������͡�)��Һ��
����20��ʱ����NH4HCO3 21 g�ı�����Һ�м�������ʳ�Ρ�����ʳ�εļ��룬�������ľ�����____��
A.NH4HCO3 B. NH4Cl C.NaHCO3
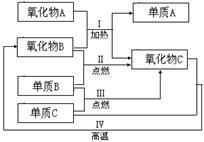
��1����Ԫ�غ���Ԫ�� ����
��2��2NaCl + 2H2O 2NaOH + H2�� + Cl2�� ����
2NaOH + H2�� + Cl2�� ����
��3���� NH4Cl �� ���� �� C
��2��2NaCl + 2H2O
 2NaOH + H2�� + Cl2�� ����
2NaOH + H2�� + Cl2�� ������3���� NH4Cl �� ���� �� C
�����������1�����ˮʵ��֤���ˣ�ˮ������Ԫ�غ���Ԫ����ɵġ������Ƕȿ���ˮ��ˮ���ӹ��ɡ�
��2����ⱥ���Ȼ�����Һ�Ļ�ѧ����ʽΪ2NaCl + 2H2O
 2NaOH + H2�� + Cl2�����÷�Ӧ�е���ת��Ϊ��ѧ�ܡ�
2NaOH + H2�� + Cl2�����÷�Ӧ�е���ת��Ϊ��ѧ�ܡ���3���� �������ܽ�����ݿɵã�30��ʱ�ܽ��������NH4Cl��
��10��ʱ��̼����淋��ܽ����15.8g��������16.8gNH4HCO3�������100gˮ�г���ܽ⣬������ҺΪ������Һ��
����NH4HCO3����Һ�м�������ʳ�Σ�����Ϊ��Һ�д��ڵ������У�NH4HCO3 ��NH4Cl��NaHCO3��NaCI����20��ʱ�������ʵ��ܽ�ȿ�֪��NaHCO3���ܽ����С������20��ʱ������NH4HCO3 21 g�ı�����Һ�м�������ʳ�Ρ�����ʳ�εļ��룬�������ľ�����NaHCO3��
��������NH4HCO3����Һ�м�������ʳ�Σ�����Ϊ��Һ�д��ڵ������У�NH4HCO3 ��NH4Cl��NaHCO3��NaCI�����ǽ��С��Ĺؼ���

��ϰ��ϵ�д�
 С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д� һ������ϵ�д�
һ������ϵ�д� �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д�
�����Ŀ