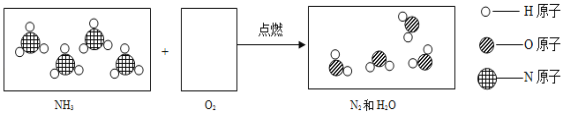
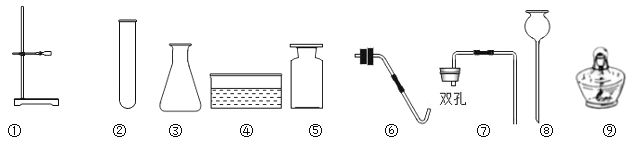
��Ŀ����
����Ŀ���������ѧ֪ʶ�ش��������⣺
(1)��Ȼ������Ӳ��������_______(������)��
(2)ʳ�����к��������ӣ��뻭�������ӵĽṹʾ��ͼ_______��
(3)���״���ʯ����ֽ�ϣ���ֽ����ɫ��Ϊ_______ɫ��
(4)���Ȳ��˵ij���������______���������ЧӦ����Ҫ������_______������Ѫ�쵰�������������ж���������_______(��С��ȫ���ѧʽ)��
(5)�ؿ��к������Ľ���Ԫ����ǽ���Ԫ���γɵĻ�������_______(�ѧʽ)��
(6)Ӳˮ����ˮ����_______�����֣�����Ӳˮ�ķ����ܶ࣬�������г���_______������ˮ�ľ��������У�������__(������)�������Գ�ȥˮ����ζ��
���𰸡����ʯ 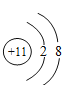 �� O2 CO2 CO Al2O3 ����ˮ ������� ����̿
�� O2 CO2 CO Al2O3 ����ˮ ������� ����̿
��������
��1�����ʯ��Ŀǰ�ڵ����Ϸ��ֵ��ڶ���Ȼ���ڵ����������Ӳ�����ʣ�
��2����Ԫ����11��Ԫ�أ�������11�����ӣ�������11�����ӣ��ڻ�ѧ��Ӧ����ʧȥ������һ�����Ӷ��γ������ӣ����� 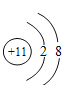 ��
��
��3���״��к��д��ᣬ�����ԣ���ʹʯ����ֽ���ɫ��
��4�����Ȳ��˵ij�����������������ѧʽ��O2��������̼���������ЧӦ����Ҫ���壬��ѧʽ��CO2��һ����̼����Ѫ�쵰��ϣ������룬��������ж������壬��ѧʽΪCO��
��5���ؿ��к�������ǰ����Ԫ�أ������衢���������ƣ����Եؿ��к������Ľ���Ԫ���������ǽ���Ԫ��Ϊ�����γɵĻ�����ΪAl2O3��
��6�������г��÷���ˮ��������ˮ��Ӳˮ���������ˮ�����ݛi�ḻ������ˮ����ĭ�ٵ���Ӳˮ�������г��ü�����еķ�������Ӳˮ������̿���������ԣ������ھ�ˮ������������ζ��ɫ�ء�