��Ŀ����
��֪����NH4HCO3
NH3��+CO2��+H2O����2NH3+H2SO4=��NH4��2SO4����2NH3+3CuO
3Cu+N2+3H2O���ܰ����ܶ�С�ڿ�������������ˮ������ǿ�ҵĴ̼�����ζ���ǿ�������Ⱦ��֮һ��
ijͬѧ���������װ�ã��ô���������İ�����ԭ����ͭ����ͨ���ⶨ����ˮ����������ͭ�����ԭ������
�Իش�
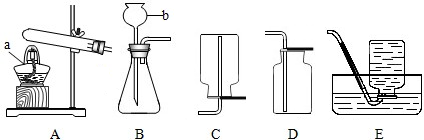
��1�����������a b
��2����ȷ��װ������˳��Ϊ��A���� E ����B����D ����C
��3��Eװ�õ������� Cװ�õ�������
��4��ʵ����ȷ��ȡmg CuO����ַ�Ӧ�������ˮ������Ϊng����ͭ�����ԭ������Ϊ �����û��Eװ�ã�ʵ�ʲ�õĽ�� ���ƫ����ƫС����
��5����֪������ͭ��Cu2O����Ҳ�Ǻ�ɫ������ַ�Ӧ�����ɵIJ���ֻ��ͭ����Ӧ�����ʱ����������ͭ���ɣ�������ͭ��ϡ���ᷴӦʱ��������Cu2+��Cu��д��������ͭ��ϡ���ᷴӦ�Ļ�ѧ����ʽ
��6����Ӧ��ĺ�ɫ��ĩ������²����ijɷ֣�����Ƽ�Ҫʵ��֤����IJ²�
| ||
| ||
ijͬѧ���������װ�ã��ô���������İ�����ԭ����ͭ����ͨ���ⶨ����ˮ����������ͭ�����ԭ������
�Իش�

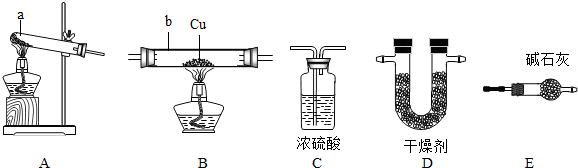
��1�����������a
��2����ȷ��װ������˳��Ϊ��A���� E ����B����D ����C
��3��Eװ�õ�������
��4��ʵ����ȷ��ȡmg CuO����ַ�Ӧ�������ˮ������Ϊng����ͭ�����ԭ������Ϊ
��5����֪������ͭ��Cu2O����Ҳ�Ǻ�ɫ������ַ�Ӧ�����ɵIJ���ֻ��ͭ����Ӧ�����ʱ����������ͭ���ɣ�������ͭ��ϡ���ᷴӦʱ��������Cu2+��Cu��д��������ͭ��ϡ���ᷴӦ�Ļ�ѧ����ʽ
��6����Ӧ��ĺ�ɫ��ĩ������²����ijɷ֣�����Ƽ�Ҫʵ��֤����IJ²�
| �� �� | �� Ҫ ʵ �� �� �� | �� �� | �� �� |
��������dz������ʵ����ƣ��˽ⳣ�����ʵ���;��֪�����ݷ���ʽ����IJ��裮
����⣺��1���˽ⳣ�����ʵ����ƺ���;��
��2��װ������˳��Ҫ�����ô���������İ�����ԭ����ͭ����ͨ���ⶨ����ˮ����������ͭ�����ԭ��������
��3����ʯ�����������ƺ������ƵĻ�����������ˮ��Ҳ�����ն�����̼���������壻
��4�����ݷ���ʽ�б���ʽ���м��㣬û��Eװ�ã�����ˮ�������ͻ�ƫ�࣬���ݱ���ʽ��֪ͭ�����ԭ����������ƫС��
��5�����������֪��Ӧ��Ϊ������ͭ��ϡ���ᣬ������Ϊͭ������ͭ���������غ㶨�ɿ�֪�������л���Ҫ��ˮ������ƽ��
��6�����������֪һ����ͭ��������������ͭ���ټ������ῴ�Ƿ���Һ�����������������������ͭ������û�У�
�ʴ�Ϊ����1���Թܣ������ܣ�
��2��E D��
��3����ȥCO2��H2O�����ն���İ�������ֹ��Ⱦ������
��4��
-16ƫС
��5��Cu2O+H2SO4=Cu+CuSO4+H2O
��6������һ��Cu��������ϡ���ᣬ��Һ����ɫ
�������Cu2O��Cu��������ϡ���ᣬ��Һ����ɫ��
��2��װ������˳��Ҫ�����ô���������İ�����ԭ����ͭ����ͨ���ⶨ����ˮ����������ͭ�����ԭ��������
��3����ʯ�����������ƺ������ƵĻ�����������ˮ��Ҳ�����ն�����̼���������壻
��4�����ݷ���ʽ�б���ʽ���м��㣬û��Eװ�ã�����ˮ�������ͻ�ƫ�࣬���ݱ���ʽ��֪ͭ�����ԭ����������ƫС��
��5�����������֪��Ӧ��Ϊ������ͭ��ϡ���ᣬ������Ϊͭ������ͭ���������غ㶨�ɿ�֪�������л���Ҫ��ˮ������ƽ��
��6�����������֪һ����ͭ��������������ͭ���ټ������ῴ�Ƿ���Һ�����������������������ͭ������û�У�
�ʴ�Ϊ����1���Թܣ������ܣ�
��2��E D��
��3����ȥCO2��H2O�����ն���İ�������ֹ��Ⱦ������
��4��
| 18m |
| n |
��5��Cu2O+H2SO4=Cu+CuSO4+H2O
��6������һ��Cu��������ϡ���ᣬ��Һ����ɫ
�������Cu2O��Cu��������ϡ���ᣬ��Һ����ɫ��
������֪���������ʵ����ƺ���;���ô���������İ�����ԭ����ͭ����ͨ���ⶨ����ˮ����������ͭ�����ԭ��������Ҫע���ȳ�ȥ�����е����ʣ�����ȥ������̼��ˮ������ͭ��������ͭ��������

��ϰ��ϵ�д�
�����Ŀ
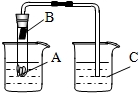 ��ͼ��С��ͬѧ��Ƶ�һ����֤NH4HCO3���ȷֽ⼰�ֽ�����װ�ã���֪NH4HCO3
��ͼ��С��ͬѧ��Ƶ�һ����֤NH4HCO3���ȷֽ⼰�ֽ�����װ�ã���֪NH4HCO3