��Ŀ����
����Ŀ����������������벻���������ϣ�
��1�������dz��õĺϽ�������������������������
��2��ij��ȤС����̽��Fe��Cu��Ag��R���ֽ����Ļ��˳��RΪδ֪������������������ʵ�飺 
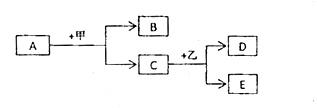
����ͼA��ʾ�����ĸ�����˿ͬʱ�����ձ��е���Һ�У����з�����Ӧ�ķ���ʽΪ ��
��һ��ʱ����ձ�����˿�滻ΪR����ʵ�飬��ͼB��ʾ�������г������ݣ��������������ɵó�Fe��Cu��Ag��R�Ļ��˳����ǿ����Ϊ ��
���𰸡�
��1�������
��2��Fe+CuSO4�TFeSO4+Cu��Fe��R��Cu��Ag
���������⣺��1����������Ҫ�ɷ�������������һЩ���ʣ����ڻ�����2������ͼA��ʾ��֪�����ĸ�����˿ͬʱ�����ձ��У�����Fe������ͭ��Ӧ��������������ͭ����Ӧ�Ļ�ѧ����ʽΪ��Fe+CuSO4�TFeSO4+Cu����һ��ʱ����ձ�����˿�滻ΪR����ʵ�飬���ڼ��г������ݣ�˵����R�ڽ������˳����У�λ�����ǰ�ߣ��������������������ձ����е�������FeSO4 �� ˵��R�Ļ�����С�������ɵó�Fe��Cu��Ag��R�Ļ��˳����ǿ����Ϊ��Fe��R��Cu��Ag�� ���Դ��ǣ���1��������2����Fe+CuSO4�TFeSO4+Cu����Fe��R��Cu��Ag��
�����㾫����������Ĺؼ���������������˳����Ӧ�õ����֪ʶ�������ڽ������˳���1��������λ��Խ��ǰ�����Ļ�Ծ�Խǿ2��λ����ǰ��Ľ������û������ᡢϡ�����е��⣨������Ũ���ᡢ���ᣩ3��λ��ǰ��Ľ����ܰ�λ�ں���Ľ��������ǵ�����Һ���û�����������K��Ca��Na�����Լ�����д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ�����⣬�˽�ע�⣺a����ƽ b������ c�����ţ�