��Ŀ����
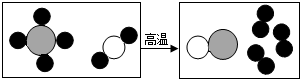
 ��2012?���У���ͼ��ʾ��A��D�Ǿ��꼶��ѧ�������ʣ���֪AΪ���������CΪ����ʯ����Ҫ�ɷ֣���������ʾ����֮����ڵ�ת����ϵ������������δ���������ش��������⣺
��2012?���У���ͼ��ʾ��A��D�Ǿ��꼶��ѧ�������ʣ���֪AΪ���������CΪ����ʯ����Ҫ�ɷ֣���������ʾ����֮����ڵ�ת����ϵ������������δ���������ش��������⣺��1��D��������
����
����
������һ�ּ��ɣ���2��B��D֮��Ļ�ѧ��Ӧ����
���ֽ�
���ֽ�
��Ӧ���������Ӧ���ͣ�����3��д��A��B�Ļ�ѧ����ʽ
CaO+H2O=Ca��OH��2
CaO+H2O=Ca��OH��2
����4��д��B�����ɡ������е�һ����;
���������
���������
������������CΪ����ʯ����Ҫ�ɷ֣���֪CΪ̼��ƣ�C���·ֽ�����A����AΪ�����������AΪ�����ƣ�A��ˮ��Ӧ��������B��BΪ�������ƣ�����B��D��Ӧ��IJ���Ϊ̼��ƺ��������ƣ������ƶ�D��̼���ƣ��ݴ˽��з�����ɣ�
����⣺CΪ����ʯ����Ҫ�ɷ֣���֪CΪ̼��ƣ�C���·ֽ�����A����AΪ�����������AΪ�����ƣ�A��ˮ��Ӧ��������B��BΪ�������ƣ�����B��D��Ӧ��IJ���Ϊ̼��ƺ��������ƣ������ƶ�D��̼���ƣ�
��1��DΪ̼���ƣ��׳ƴ�����մ�
��2��BΪ�������ƣ�DΪ̼���ƣ����߷�Ӧ����̼��Ƴ������������ƣ������ֻ�����������ɷ������������ֻ�����ķ�Ӧ�����ڸ��ֽⷴӦ��
��3��AΪ�����ƣ���ˮ��Ӧ�����������ƣ���Ӧ�Ļ�ѧ����ʽΪ��CaO+H2O=Ca��OH��2��
��4��BΪ�������ƣ������ɡ������п������к��������������������ϵȣ�
�ʴ�Ϊ����1�������2�����ֽ⣻��3��CaO+H2O=Ca��OH��2����4���к����������ȣ�
��1��DΪ̼���ƣ��׳ƴ�����մ�
��2��BΪ�������ƣ�DΪ̼���ƣ����߷�Ӧ����̼��Ƴ������������ƣ������ֻ�����������ɷ������������ֻ�����ķ�Ӧ�����ڸ��ֽⷴӦ��
��3��AΪ�����ƣ���ˮ��Ӧ�����������ƣ���Ӧ�Ļ�ѧ����ʽΪ��CaO+H2O=Ca��OH��2��
��4��BΪ�������ƣ������ɡ������п������к��������������������ϵȣ�
�ʴ�Ϊ����1�������2�����ֽ⣻��3��CaO+H2O=Ca��OH��2����4���к����������ȣ�
����������Ϊ��ͼ�������ƶ��⣬����Ĺؼ����������ͻ�ƿڣ�Ȼ��˳�ƻ����ƻ����������м��Ƶó��������ʵĻ�ѧʽ��

��ϰ��ϵ�д�
 ���Ǽ���С����ϵ�д�
���Ǽ���С����ϵ�д� �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д�
�����Ŀ