��Ŀ����
��14�֣���������ʵ�鳣��װ�ã��ش��й����⡣
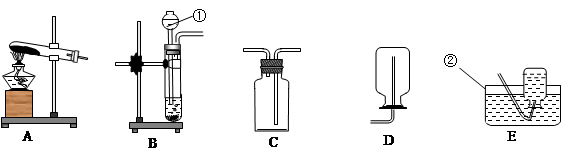
��1��д��ͼ�б�����ĸ�����������ƣ�a ��
��2��ʵ�����ø��������ȡ������Ӧѡ�õķ���װ�� ����Ӧ�Ļ�ѧ����ʽ�� ��
��3���Ƚ��ù���������Һ���ø��������ȡO2�ķ��������ߵĹ�ͬ���� ����д��ţ���
��4��ʵ������ȡ������̼�ķ�Ӧ����ʽΪ ����Ӧװ��Ӧѡ�� ��ʵ��ʱӦ�� �� ����װ��ҩƷ�ڼ��������ԣ��������Eװ���ռ������壬������� �˽��루�b����c������
��5��ʵ���ҳ���װ��C����װ��B��ȡ���壬��װ�õ��ŵ���
�� ���з�Ӧ�����ڸ�װ�õ��� ������ţ� ��
�ٴ���ʯ��ϡ���� ��̼���ƹ����ϡ����
��п����ϡ���� �ܹ���������Һ���������
��6����ͬѧ���������ͼ��ʾʵ������ȡCO2װ�ã���װ���Ƿ���� C װ�õ����ã���ǡ�����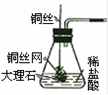

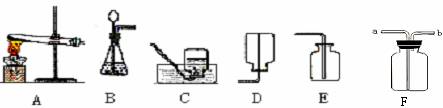
��1��д��ͼ�б�����ĸ�����������ƣ�a ��
��2��ʵ�����ø��������ȡ������Ӧѡ�õķ���װ�� ����Ӧ�Ļ�ѧ����ʽ�� ��
��3���Ƚ��ù���������Һ���ø��������ȡO2�ķ��������ߵĹ�ͬ���� ����д��ţ���
| A������װ����ͬ | B��������MnO2������ |
| C����Ӧ�Ļ���������ͬ | D����ȫ��Ӧ��ʣ�����ɷ���ͬ |
��5��ʵ���ҳ���װ��C����װ��B��ȡ���壬��װ�õ��ŵ���
�� ���з�Ӧ�����ڸ�װ�õ��� ������ţ� ��
�ٴ���ʯ��ϡ���� ��̼���ƹ����ϡ����
��п����ϡ���� �ܹ���������Һ���������
��6����ͬѧ���������ͼ��ʾʵ������ȡCO2װ�ã���װ���Ƿ���� C װ�õ����ã���ǡ�����

��1������©����2��A 2KMnO4 K2MnO4 + MnO2 + O2����3��C
K2MnO4 + MnO2 + O2����3��C
��4��CaCO3 + 2HCl = CaCl2 + H2O +CO2�� B��C �� �� b
��5�����Կ��Ʒ�Ӧ��ʼ��ֹͣ �٢� ��6����
 K2MnO4 + MnO2 + O2����3��C
K2MnO4 + MnO2 + O2����3��C��4��CaCO3 + 2HCl = CaCl2 + H2O +CO2�� B��C �� �� b
��5�����Կ��Ʒ�Ӧ��ʼ��ֹͣ �٢� ��6����
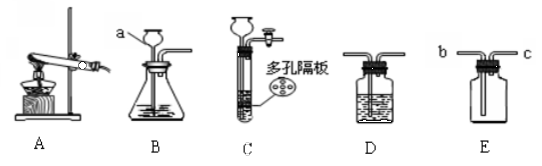
��1������ʵ���ҳ���������������գ�
��2��ʵ�����ø��������ȡ����Ӧ��ѡȡ����+�� �����͡���ȡװ�ã����ݸ÷�Ӧ�ķ�Ӧ��������Լ���Ӧ������ѧ��ѧ��Ӧ����ʽ��
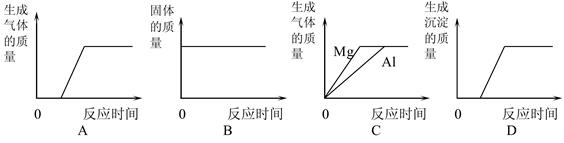
��3���ɸ���������ȡ�����ķ�Ӧԭ�����жԱȣ�
��4����ʵ�����ƶ�����̼��ԭ����ȷд����ѧ����ʽ��Ũ���������ˮ�ԣ�������������ܸ�������������������壻
��5������Cװ����Bװ�õ�ʹ��ԭ���ͷ����ش�װ��C�ʺϿ�״������Һ�巴Ӧ��
��2��ʵ�����ø��������ȡ����Ӧ��ѡȡ����+�� �����͡���ȡװ�ã����ݸ÷�Ӧ�ķ�Ӧ��������Լ���Ӧ������ѧ��ѧ��Ӧ����ʽ��
��3���ɸ���������ȡ�����ķ�Ӧԭ�����жԱȣ�
��4����ʵ�����ƶ�����̼��ԭ����ȷд����ѧ����ʽ��Ũ���������ˮ�ԣ�������������ܸ�������������������壻
��5������Cװ����Bװ�õ�ʹ��ԭ���ͷ����ش�װ��C�ʺϿ�״������Һ�巴Ӧ��

��ϰ��ϵ�д�
 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
�����Ŀ