��Ŀ����
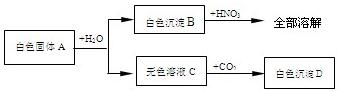
34����һ����ɫ����A�����ܺ���FeCl3��CaCO3��NaOH��Ba��OH��2��BaCl2��Na2SO4�еļ��֣�ȡ����A������ʵ�飬������ͼ��ʾ�����û�ѧʽ��գ�
��1����ɫ����B��
��2����ɫ����A��һ�������ڵ�����Ϊ
��3������ɫ����A��ֻ���������ʣ�������
��4����ɫ����A������Щ���ܵ���ɣ���
��
��1����ɫ����B��
CaCO3
D��
BaCO3
����2����ɫ����A��һ�������ڵ�����Ϊ
FeCl3��Na2SO4
����3������ɫ����A��ֻ���������ʣ�������
CaCO3��Ba��OH��2
����4����ɫ����A������Щ���ܵ���ɣ���
CaCO3��Ba��OH��2
��CaCO3��Ba��OH��2��NaOH
��
CaCO3��Ba��OH��2��BaCl2
��CaCO3��Ba��OH��2��NaOH��BaCl2
��
���������ں�Fe3+��Һ�ʻ�ɫ�������Ϊ���ɫ�����ݰ�ɫ����A����ˮ�õ���ɫ����B����ɫ��ҺC����ȷ����ɫ����A�в���FeCl3�����ݰ�ɫ����Bȫ������ϡ���ᣬ���жϰ�ɫ����ΪCaCO3����ɫ����A��Ba��OH��2��BaCl2��Na2SO4����ͬʱ���ڣ��ڼ������ʵ���Һ�У���������CO2��Ӧ�γɳ�����ֻ��Ba��OH��2����ˣ���ɫ����A��һ������Ba��OH��2���������Ϸ�������ɫ����A�Ŀ���������������ƶϣ�����������Ľ��
����⣺��1�����ݰ�ɫ����B����ȫ���ܽ���ϡ���ᣬ��Ϲ���A�Ŀ�����ɣ����жϳ���BΪ̼��ƣ����ݹ���A�Ŀ�����ɣ�����Щ���ܺ��е������У�ֻ��Ba��OH��2����CO2��Ӧ����BaCO3����������ȷ������DΪBaCO3��
�ʴ�Ϊ��CaCO3��BaCO3��
��2�����ݰ�ɫ����B����ȫ���ܽ���ϡ���ᣬ�����жϰ�ɫ����A��Ba��OH��2��BaCl2��Na2SO4����ͬʱ���ڣ�������һ������Ba��OH��2�����ԣ�����A��һ������Na2SO4�����ݰ�ɫ����A����ˮ�õ���ɫ����B����ɫ��ҺC����ɫ����A��һ������FeCl3��
�ʴ�Ϊ��FeCl3��Na2SO4��
��3������A��һ������CaCO3��Ba��OH��2�����ԣ�����ɫ����A��ֻ���������ʣ�������CaCO3��Ba��OH��2��
�ʴ�Ϊ��CaCO3��Ba��OH��2��
��4����ɫ����A��һ������CaCO3��Ba��OH��2��һ������FeCl3��Na2SO4�����ܺ���NaOH��BaCl2�����ɫ����A�Ŀ���������������֣�CaCO3��Ba��OH��2��CaCO3��Ba��OH��2��NaOH��CaCO3��Ba��OH��2��BaCl2��CaCO3��Ba��OH��2��NaOH��BaCl2��
�ʴ�Ϊ��CaCO3��Ba��OH��2��CaCO3��Ba��OH��2��NaOH��CaCO3��Ba��OH��2��BaCl2��CaCO3��Ba��OH��2��NaOH��BaCl2��
�ʴ�Ϊ��CaCO3��BaCO3��
��2�����ݰ�ɫ����B����ȫ���ܽ���ϡ���ᣬ�����жϰ�ɫ����A��Ba��OH��2��BaCl2��Na2SO4����ͬʱ���ڣ�������һ������Ba��OH��2�����ԣ�����A��һ������Na2SO4�����ݰ�ɫ����A����ˮ�õ���ɫ����B����ɫ��ҺC����ɫ����A��һ������FeCl3��
�ʴ�Ϊ��FeCl3��Na2SO4��
��3������A��һ������CaCO3��Ba��OH��2�����ԣ�����ɫ����A��ֻ���������ʣ�������CaCO3��Ba��OH��2��
�ʴ�Ϊ��CaCO3��Ba��OH��2��
��4����ɫ����A��һ������CaCO3��Ba��OH��2��һ������FeCl3��Na2SO4�����ܺ���NaOH��BaCl2�����ɫ����A�Ŀ���������������֣�CaCO3��Ba��OH��2��CaCO3��Ba��OH��2��NaOH��CaCO3��Ba��OH��2��BaCl2��CaCO3��Ba��OH��2��NaOH��BaCl2��
�ʴ�Ϊ��CaCO3��Ba��OH��2��CaCO3��Ba��OH��2��NaOH��CaCO3��Ba��OH��2��BaCl2��CaCO3��Ba��OH��2��NaOH��BaCl2��
�������������ʵ����ʼ��仯���ɣ����ת���仯��ͼ�е�ʵ������ȷ��������һ�����е�������һ�������е����ʣ�Ȼ��Կ��ܺ��е����ʽ�����ϣ�

��ϰ��ϵ�д�
�����Ŀ
 ��2005?��������1������������һ����Ҫ�Ļ���ԭ�ϣ�������ɱ�������ˮ����ľ�ķ�������������������ȱ����ƶѪ����ҵ���÷���м��ϡ���ᷴӦ��������������Ҫ��Ӧ�Ļ�ѧ����ʽΪ
��2005?��������1������������һ����Ҫ�Ļ���ԭ�ϣ�������ɱ�������ˮ����ľ�ķ�������������������ȱ����ƶѪ����ҵ���÷���м��ϡ���ᷴӦ��������������Ҫ��Ӧ�Ļ�ѧ����ʽΪ