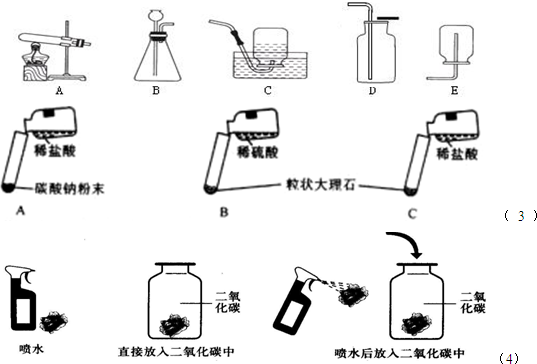
��Ŀ����
��ͼ��ʵ���ҳ��õ�������ȡ���ռ�װ�ã�����ش��������⣺
��1��д�����б�ŵ��������ƣ��� ���� ��
��2��ʵ������ȡ���ռ�CO2��Ӧѡ�õ�װ���ǣ����ţ� ����Ӧ�Ļ�ѧ����ʽΪ ��
��3��С��ѡ��B��Dװ�ã��ù�������Ͷ���������ȡһƿO2��Ϊ�˽�ԼҩƷ����������²�������������Bװ���м��������������̣�Ȼ�������������μ������������Һ�����պ�����һƿO2�����IJ�������������������������� �������� ��
��1������©����ˮ�ۣ���1�֣���2�֣�
��2��BC����1�֣���2�֣��� CaCO3 + 2HCl ="===" CaCl2 + H2O + CO2����2�֣�
��3����������1�֣��������Һ��δ�ܷ�ס����©���¶˵Ĺܿڣ�����������ͻ�ӳ���©���ų���1�֣�����:
��
��2��BC����1�֣���2�֣��� CaCO3 + 2HCl ="===" CaCl2 + H2O + CO2����2�֣�
��3����������1�֣��������Һ��δ�ܷ�ס����©���¶˵Ĺܿڣ�����������ͻ�ӳ���©���ų���1�֣�����:
��

��ϰ��ϵ�д�
 ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д� ���ɿ��ñ���ϵ�д�
���ɿ��ñ���ϵ�д�
�����Ŀ