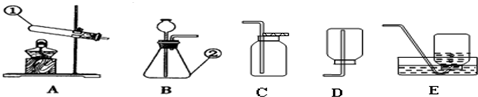
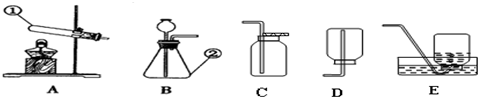
��Ŀ����
��1��ע�ⰲȫ���۾��ヲ����ҩҺ�����Ӧ����ʩ��
��2����ԼҩƷ��ȡ��ҩƷʱ�����û��˵��������һ����Һ��ȡ
��3���淶��������������ƽ����ҩƷʱ���������
2�������ܲ�����Ƥ��ʱ������ˮ��ʪ��������Ȼ��������ת������Ƥ���У�
3��ȡ��ҩƷʱ�����û��˵��������һ��Һ��ҩƷȡ1-2ml������ҩƷֻ������Թܵײ���
4��ʹ��������ƽʱ��Ӧע�������������ң�
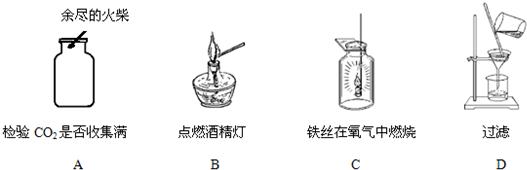
5�����������̼�Ƿ��ռ����ķ����ǣ���ȼ�ŵ�ľ�����ڼ���ƿ�ڣ�
6��ʹ�þƾ��ƣ�Ҫ�û���ȼ�ƾ��ƣ����Խ�ֹ��ȼ�ŵľƾ���ȥ��ȼ��һյ�ƾ��ƣ�
7����ϸ��˿����������ȼ�գ�����ƿ�ײ�Ӧ��װ����ˮ����ֹ�����ヲ��ը�Ѽ���ƿ��
8�����˹��̵IJ���Ҫ���ǡ�һ��������������©���¶˹ܿ�Ҫ�����ձ��ڱڣ���ֹ��Һ������
��2����ԼҩƷ��ȡ��ҩƷʱ�����û��˵��������һ����Һ��ȡ1-2ml������ȡ���Ǹ����Թܵײ����ɣ�
��3���淶��������������ƽ����ҩƷʱ����Ʒ�������̣�����������̣�
���ĸ�ʵ�����ͼ�У�A��ȼ�ŵ�ľ��������ڼ���ƿ�ڣ�A����B��ʹ�û���ȼ�ƾ��ƣ�B��ȷ��C����˿��������ȼ�գ�����ƿ�ײ�Ԥ�ȼ�����ˮ��C����D�����˹����У�©���¶˹ܿ�Ҫ�����ձ��ڱڣ�D����
�ʴ��ǣ�
��1�������ô�����ˮ��ϴ����ϴ��գ�۾���ʪ������
��2��1-2�������Թܵײ����ң�B

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���10�֣���ѧʵ����ѧϰ��ѧ�Ļ��������������װ��ͼ�ش����⡣
��1��Aͼ����ȡCO2�Ļ�ѧ����ʽΪ �� ��Ũ����������� �� ��
��2�����ӿ�a��b���ӣ��ɹ۲쵽���������沣������ʪ���ʯ����ֽ��죬���沣����������������������ݴ�˵�����ٶ�����̼�����ܶȱȿ����ܶ� �� ���ڶ�����̼��ˮ��Ӧ������ �� �����ʡ�
��3����֪����CaCO3+CO2+H2O= Ca(HCO3)2����Ca(HCO3)2������ˮ���������ʯ��ˮ��̼������Һ��Ӧ������̼��Ƴ�����Ҳ�������ᷴӦ�ų�������̼���塣
���ӿ�a��c���ӣ�ͨ��һ�����Ķ�����̼������Թܾ��ã��ǹ�Һ���룬���ϲ���Һ�п��ܺ��е������� �� �� �� ���������ʵ�鷽��������֤ ��
| ʵ����� | ʵ������ | ���� |
| ȡ�����ϲ���Һ���Թ��У���Ȼ��μ� �� | �� | ����Ϊ �� |
��9�֣���ѧʵ����ѧϰ��ѧ�Ļ��������������װ��ͼ�ش����⡣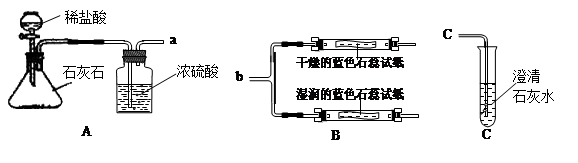 ��1��Aͼ����ȡCO2�Ļ�ѧ����ʽΪ ��Ũ����������� ��
��1��Aͼ����ȡCO2�Ļ�ѧ����ʽΪ ��Ũ����������� ��
��2�����ӿ�a��b���ӣ��ɹ۲쵽���������沣������ʪ���ʯ����ֽ��죬���沣����������������������ݴ�˵�����ٶ�����̼�����ܶȱȿ����ܶ� ���ڶ�����̼��ˮ��Ӧ������ �����ʡ�
��3����֪����CaCO3+CO2+H2O= Ca��HCO3��2����Ca��HCO3��2������ˮ���������ʯ��ˮ��̼������Һ��Ӧ������̼��Ƴ�����Ҳ�������ᷴӦ�ų�������̼���塣���ӿ�a��c���ӣ�ͨ��һ�����Ķ�����̼������Թܾ��ã��ǹ�Һ���룬���ϲ���Һ�п��ܺ��е�������Ca(OH)2�� ���������ʵ�鷽��������֤��
| ʵ����� | ʵ������ | ���� |
| ȡ�����ϲ���Һ���Թ��У�Ȼ��μ� | | ���ʺ��� |
��ѧʵ����ѧϰ��ѧ�Ļ��������������װ��ͼ�ش����⡣
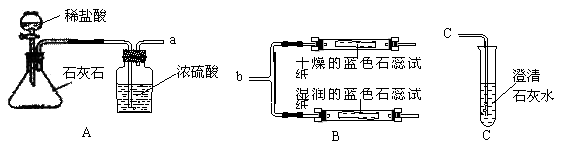 |
��1��Aͼ����ȡCO2�Ļ�ѧ����ʽΪ ��Ũ����������� ��
��2�����ӿ�a��b���ӣ��ɹ۲쵽���������沣������ʪ���ʯ����ֽ��죬���沣����������������������ݴ�˵�����ٶ�����̼�����ܶȱȿ����ܶ� ���ڶ�����̼��ˮ��Ӧ������ �����ʡ�
��3����֪����CaCO3+CO2+H2O= Ca��HCO3��2����Ca��HCO3��2 ������ˮ���������ʯ��ˮ��̼������Һ��Ӧ������̼��Ƴ�����Ҳ�������ᷴӦ�ų�������̼���塣���ӿ�a��c���ӣ�ͨ��һ�����Ķ�����̼������Թܾ��ã��ǹ�Һ���룬���ϲ���Һ�п��ܺ��е�������Ca(OH)2�� ���������ʵ�鷽��������֤ ��
| ʵ����� | ʵ������ | ���� |
| ȡ�����ϲ���Һ���Թ��У�Ȼ��μ� |
| ���ʺ��� |