题目内容
按要求用正确的化学符号表示:
(1)2个氮原子______ (2)m个铵根离子______
(3)正二价的汞元素______ (4)1个硫酸分子______
(5)氧化镁中镁元素为+2价______ (6)3个亚铁离子______
(7)构成氯化钠晶体的阴离子______ (8)由硫离子与亚铁离子构成的物质______
(9)地壳里含量最多的元素是______,含量最多的金属元素是______,二者组成的化合物的化学式是______,空气中体积分数最大的气体是______,能使带火星的木条复燃的气体是______,该气体占空气体积的______,常温下密度最小的气体是______.
解:(1)原子的表示方法就是用元素符号来表示一个原子,表示多个该原子,就在其元素符号前加上相应的数字.所以2个氮原子,就可表示为 2N
(2)离子的表示方法:在表示该离子的元素符号右上角,标出该离子所带的正负电荷数,数字在前,正负符号在后,带1个电荷时,1要省略.表示多个离子在离子符号前面加数字,故m个铵根离子符号为:mNH4+
(3)元素的化合价的正确标法是在元素符号或原子团符号的正上方标明化合价的种类和价目,+2价的汞元素符号为
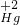
(4)分子的表示方法:正确书写物质的化学式,表示多个该分子,就在其化学式前加上相应的数字,因此一个硫酸分子表示为:H2SO4;
(5)元素的化合价的正确标法是在元素符号或原子团符号的正上方标明化合价的种类和价目,氧化镁中镁元素的化合价为+2价,符号为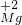 O
O
(6)离子的表示方法:在表示该离子的元素符号右上角,标出该离子所带的正负电荷数,数字在前,正负符号在后,带1个电荷时,1要省略.表示多个离子在离子符号前面加数字,故三个亚铁离子符号为3Fe2+
(7)氯化钠是由钠离子和氯离子构成的,阴离子符号为Cl-
(8)硫离子和亚铁离子构成的物质为硫化亚铁,符号为FeS
(9)地壳里含量最多的元素是氧元素,符号为 O,含量最多的金属元素是铝元素,符号为 Al,二者组成的化合物的化学式是 Al2O3,空气中体积分数最大的气体是氮气,符号为 N2,能使带火星的木条复燃的气体是氧气,符号为 O2,该气体占空气体积的 21%,常温下密度最小的气体是氢气,符号为H2
故答案为:(1)2N (2)mNH4+(3)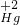 (4)H2SO4(5)
(4)H2SO4(5)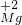 O (6)3Fe2+(7)Cl- (8)FeS(9)O,Al,Al2O3,N2,O2,21%,H2
O (6)3Fe2+(7)Cl- (8)FeS(9)O,Al,Al2O3,N2,O2,21%,H2
分析:本题考查化学用语的意义及书写,解题关键是分清化学用语所表达的对象是分子、原子、离子还是化合价,才能在化学符号前或其它位置加上适当的计量数来完整地表达其意义,并能根据物质化学式的书写规则正确书写物质的化学式,才能熟练准确的解答此类题目.
点评:本题主要考查学生对化学用语的书写和理解能力,题目设计既包含对化学符号意义的了解,又考查了学生对化学符号的书写,考查全面,注重基础,题目难度较易.
(2)离子的表示方法:在表示该离子的元素符号右上角,标出该离子所带的正负电荷数,数字在前,正负符号在后,带1个电荷时,1要省略.表示多个离子在离子符号前面加数字,故m个铵根离子符号为:mNH4+
(3)元素的化合价的正确标法是在元素符号或原子团符号的正上方标明化合价的种类和价目,+2价的汞元素符号为
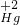
(4)分子的表示方法:正确书写物质的化学式,表示多个该分子,就在其化学式前加上相应的数字,因此一个硫酸分子表示为:H2SO4;
(5)元素的化合价的正确标法是在元素符号或原子团符号的正上方标明化合价的种类和价目,氧化镁中镁元素的化合价为+2价,符号为
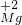 O
O(6)离子的表示方法:在表示该离子的元素符号右上角,标出该离子所带的正负电荷数,数字在前,正负符号在后,带1个电荷时,1要省略.表示多个离子在离子符号前面加数字,故三个亚铁离子符号为3Fe2+
(7)氯化钠是由钠离子和氯离子构成的,阴离子符号为Cl-
(8)硫离子和亚铁离子构成的物质为硫化亚铁,符号为FeS
(9)地壳里含量最多的元素是氧元素,符号为 O,含量最多的金属元素是铝元素,符号为 Al,二者组成的化合物的化学式是 Al2O3,空气中体积分数最大的气体是氮气,符号为 N2,能使带火星的木条复燃的气体是氧气,符号为 O2,该气体占空气体积的 21%,常温下密度最小的气体是氢气,符号为H2
故答案为:(1)2N (2)mNH4+(3)
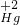 (4)H2SO4(5)
(4)H2SO4(5) O (6)3Fe2+(7)Cl- (8)FeS(9)O,Al,Al2O3,N2,O2,21%,H2
O (6)3Fe2+(7)Cl- (8)FeS(9)O,Al,Al2O3,N2,O2,21%,H2分析:本题考查化学用语的意义及书写,解题关键是分清化学用语所表达的对象是分子、原子、离子还是化合价,才能在化学符号前或其它位置加上适当的计量数来完整地表达其意义,并能根据物质化学式的书写规则正确书写物质的化学式,才能熟练准确的解答此类题目.
点评:本题主要考查学生对化学用语的书写和理解能力,题目设计既包含对化学符号意义的了解,又考查了学生对化学符号的书写,考查全面,注重基础,题目难度较易.

练习册系列答案
 备战中考寒假系列答案
备战中考寒假系列答案
相关题目
对下列事实解释错误的是
| 事实 | 解释 | |
| A | 酒香不怕巷子深 | 分子在不断运动 |
| B | 物体热胀冷缩 | 分子的体积发生改变 |
| C | CO有毒而CO2无毒 | 两种物质的分子构成不同 |
| D | 加热氧化汞得到汞和氧气 | 化学变化中,分子发生改变 |
- A.A
- B.B
- C.C
- D.D
某校进行化学实验考查时,教师给了同学们一小包黑色粉末,这种黑色粉末可能是氧化铜、炭粉或者是两者的混合物,让他们通过实验探究来确定.某同学探究过程如下:
(1)[提出假设]假设1:黑色粉末是炭粉;假设2:______;假设3:______.
(2)[设计实验方案]他对实验做了如下设想和分析:
取少量黑色粉末于烧杯中,加入过量的稀硫酸(稀硫酸与氧化铜反应生成的溶液呈蓝色,与炭不反应),充分反应后过滤.则实验中可能出现的现象与对应结论如下表,请你完成下表.
| 实验中可能出现的现象 | 结论 |
| (1)______ | ______ |
| (2)滤纸上无黑色粉末,滤液为蓝色 | 假设2成立 |
| (3)______ | 假设3成立 |
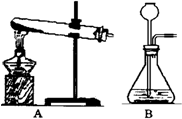 双氧水常用于消毒、漂白等,它是过氧化氢(H2O2)的水溶液.在盛有少量MnO2的容器中,加入含H2O230%的双氧水,在常温下即可迅速分解放出氧气:
双氧水常用于消毒、漂白等,它是过氧化氢(H2O2)的水溶液.在盛有少量MnO2的容器中,加入含H2O230%的双氧水,在常温下即可迅速分解放出氧气: 2H2O+O2↑.实验室可用此方法制取氧气.
2H2O+O2↑.实验室可用此方法制取氧气.