��Ŀ����
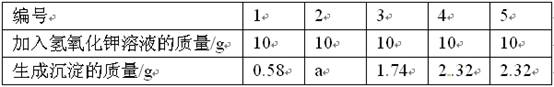
(11��)�Ȼ�þ����ȡþ��ԭ��֮һ����Ҫ�ⶨij������Ʒ(��MgCl2��KCl)���������Ȼ�þ��������������ʵ�飺�Ƚ�10g��Ʒ��ȫ����ˮ��Ȼ���50gһ����������������������Һƽ������μ�����Ʒ��Һ�У������ʵ���������ݼ��±���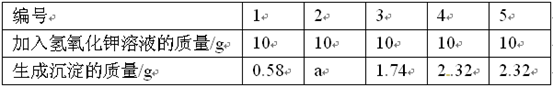
������������ݣ��ش��������⣺
(1)����aΪ______��
(2)��Ʒ�е��Ȼ������ڻ����е�______�ʣ��ɻ������á�
(3)���õ�����������Һ�����ʵ�����������______��
(4)������Ʒ���Ȼ�þ�����������Ƕ��٣�(д���������)
(11��)(1)1.16(2��) (2)��(2��) (3)11.2%(2��)
(4)��ԭ������к���MgCl2������Ϊx��
MgCl2+2KOH=Mg(OH)2��+2KCl ����������������2��
95 58
x 2.32g ����������������1��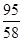 =
=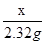 x=3.8g ����������������1��
x=3.8g ����������������1��
����Ʒ���Ȼ�þ������������ ��100%=38%����������������1��
��100%=38%����������������1��
���ԡ�
����

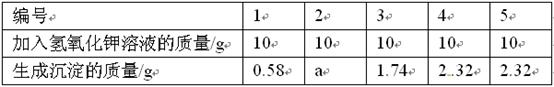
(11��)�Ȼ�þ����ȡþ��ԭ��֮һ����Ҫ�ⶨij������Ʒ(��MgCl2��KCl)���������Ȼ�þ��������������ʵ�飺�Ƚ�10g��Ʒ��ȫ����ˮ��Ȼ���50gһ����������������������Һƽ������μ�����Ʒ��Һ�У������ʵ���������ݼ��±���
|
��� |
1 |
2 |
3 |
4 |
5 |
|
��������������Һ������/g |
10 |
10 |
10 |
10 |
10 |
|
���ɳ���������/g |
0.58 |
a |
1.74 |
2.32 |
2.32 |
������������ݣ��ش��������⣺
(1)����aΪ______��(2��)
(2)��Ʒ�е��Ȼ������ڻ����е�______�ʡ�(2��)
(3)���õ�����������Һ�����ʵ�����������______��(2��)
(4)������Ʒ���Ȼ�þ�����������Ƕ��٣�(д���������)