��Ŀ����
ij����С��ͬѧ��100g�����5�μ��뵽30gʯ��ʯ��Ʒ�У� ��Ʒ�����ʼȲ�����ˮҲ�������ᷴӦ�����õ����²������ݺ�ͼ��
��1�����ϱ����ݷ�������2�μ��������aΪ______g��
��2��30gʯ��ʯ��Ʒ ��100gϡ���ᷴӦ�����ɶ�����̼______g��
��3�����5��ʵ�����ˣ�����CaCl2��������������Ϊ���٣�����ȷ��1%��
��4��10%��CaCl2��Һ����·�汣ʪ����������5��ʵ������Һ���10%��CaCl2��Һ��Ҫ����ˮ���ٿˣ�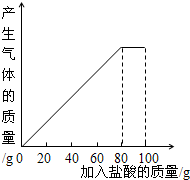
| ���� | ��һ�� | �ڶ��� | ������ |
| �������������/g | 20 | 20 | 20 |
| ʣ����������/g | 25 | a | 15 |
��2��30gʯ��ʯ��Ʒ ��100gϡ���ᷴӦ�����ɶ�����̼______g��
��3�����5��ʵ�����ˣ�����CaCl2��������������Ϊ���٣�����ȷ��1%��
��4��10%��CaCl2��Һ����·�汣ʪ����������5��ʵ������Һ���10%��CaCl2��Һ��Ҫ����ˮ���ٿˣ�

��1��ÿ����20gϡ������������������30g-25g=5g�����ԣ���2�μ���20gϡ�������ʣ����������=25g-5g=20g��
��2����4�μ���ϡ������������ܹ����ٵ�����=5g��4=20g����20g̼��������ᷴӦ���ɶ�����̼����Ϊx�������Ȼ��Ƶ�����Ϊy����
CaCO3+2HCl=CaCl2+H2O+CO2��
100 111 44
20g y x
���ݣ�
=
���x=8.8g�����ݣ�
=
���y=22.2g
��3����5��ʵ�����ˣ�����CaCl2��������������Ϊ��
��100%=20%
��4�������ܼ����ʲ��䣬�����ˮ������Ϊk��
��100%=10%�����k=110.8g
�ʴ�Ϊ����1��20����2��8.8g����3��20%����4��Ҫ����ˮ110.8g��
��2����4�μ���ϡ������������ܹ����ٵ�����=5g��4=20g����20g̼��������ᷴӦ���ɶ�����̼����Ϊx�������Ȼ��Ƶ�����Ϊy����
CaCO3+2HCl=CaCl2+H2O+CO2��
100 111 44
20g y x
���ݣ�
| 100 |
| 44 |
| 20g |
| x |
| 100 |
| 111 |
| 20g |
| y |
��3����5��ʵ�����ˣ�����CaCl2��������������Ϊ��
| 22.2g |
| 100g+20g-8.8g |
��4�������ܼ����ʲ��䣬�����ˮ������Ϊk��
| 22.2g |
| 100g+20g-8.8g+k |
�ʴ�Ϊ����1��20����2��8.8g����3��20%����4��Ҫ����ˮ110.8g��

��ϰ��ϵ�д�
�����Ŀ
ij����С��ͬѧ��100 g�����5�μ��뵽30gʯ��ʯ��Ʒ�У� ��Ʒ�����ʼȲ�����ˮҲ�������ᷴӦ�����õ����²������ݺ�ͼ�� 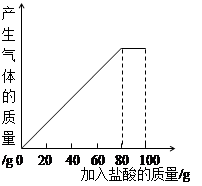
| ���� | ��һ�� | �ڶ��� | ������ |
| �������������/g | 20 | 20 | 20 |
| ʣ����������/g | 25 | a | 15 |
��2��30gʯ��ʯ��Ʒ��100gϡ���ᷴӦ�����ɶ�����̼ g��
��3�����5��ʵ�����ˣ�����CaCl2��������������Ϊ���٣�����ȷ��1%��
��4��10%��CaCl2��Һ����·�汣ʪ����������5��ʵ������Һ���10%��CaCl2��Һ��Ҫ����ˮ���ٿˣ�
ij����С��ͬѧ��100 g�����5�μ��뵽30gʯ��ʯ��Ʒ�У� ��Ʒ�����ʼȲ�����ˮҲ�������ᷴӦ�����õ����²������ݺ�ͼ��

|
���� |
��һ�� |
�ڶ��� |
������ |
|
�������������/g |
20 |
20 |
20 |
|
ʣ����������/g |
25 |
a |
15 |
��1�����ϱ����ݷ�������2�μ��������aΪ________________g��
��2��30gʯ��ʯ��Ʒ��100gϡ���ᷴӦ�����ɶ�����̼ g��
��3�����5��ʵ�����ˣ�����CaCl2��������������Ϊ���٣�����ȷ��1%��
��4��10%��CaCl2��Һ����·�汣ʪ����������5��ʵ������Һ���10%��CaCl2��Һ��Ҫ����ˮ���ٿˣ�
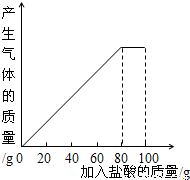
ij����С��ͬѧ��100g�����5�μ��뵽30gʯ��ʯ��Ʒ�У� ��Ʒ�����ʼȲ�����ˮҲ�������ᷴӦ�����õ����²������ݺ�ͼ��
��1�����ϱ����ݷ�������2�μ��������aΪ______g��
��2��30gʯ��ʯ��Ʒ ��100gϡ���ᷴӦ�����ɶ�����̼______g��
��3�����5��ʵ�����ˣ�����CaCl2��������������Ϊ���٣�����ȷ��1%��
��4��10%��CaCl2��Һ����·�汣ʪ����������5��ʵ������Һ���10%��CaCl2��Һ��Ҫ����ˮ���ٿˣ�
| ���� | ��һ�� | �ڶ��� | ������ |
| �������������/g | 20 | 20 | 20 |
| ʣ����������/g | 25 | a | 15 |
��2��30gʯ��ʯ��Ʒ ��100gϡ���ᷴӦ�����ɶ�����̼______g��
��3�����5��ʵ�����ˣ�����CaCl2��������������Ϊ���٣�����ȷ��1%��
��4��10%��CaCl2��Һ����·�汣ʪ����������5��ʵ������Һ���10%��CaCl2��Һ��Ҫ����ˮ���ٿˣ�

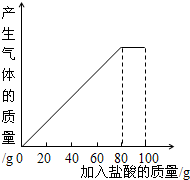 ij����С��ͬѧ��100g�����5�μ��뵽30gʯ��ʯ��Ʒ�У� ��Ʒ�����ʼȲ�����ˮҲ�������ᷴӦ�����õ����²������ݺ�ͼ��
ij����С��ͬѧ��100g�����5�μ��뵽30gʯ��ʯ��Ʒ�У� ��Ʒ�����ʼȲ�����ˮҲ�������ᷴӦ�����õ����²������ݺ�ͼ��