题目内容
我们的日常生活离不开金属.(1)地壳中含量最多的金属元素是
(2)人类从石器时代首先进入青铜器时代,请写出单质碳还原氧化铜的化学方程式
(3)据有关资料报导,每年因锈蚀而报废的金属相当于年产量的20%~40%.金属M长期露置于空气中,表面已被锈蚀.经检测,锈蚀物中除含有M外,还含有C、H、O三种元素.根据质量守恒定律推测:使金属M锈蚀的物质中一定有
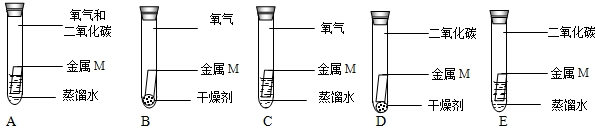
(4)小芳为验证空气中的O2是否参加了反应,设计如下实验,其中必须要做的实验是

分析:(1)根据地壳里各元素的含量由多到少的顺序排列依次是氧,硅,铝,铁,进行解答;
(2)根据化学方程式书写的要求:写配注等,写出反应的化学方程式;
(3)依据质量守恒定律中化学反应前后元素的种类不变的知识,结合空气的成分分析判断解决;
(4)设计对比试验是解决此类问题的一般思路.
(2)根据化学方程式书写的要求:写配注等,写出反应的化学方程式;
(3)依据质量守恒定律中化学反应前后元素的种类不变的知识,结合空气的成分分析判断解决;
(4)设计对比试验是解决此类问题的一般思路.
解答:解:(1)地壳中元素的含量顺序是氧硅铝铁.
故答案为:①铝(或Al);
(2)碳还原氧化铜生成铜和二氧化碳,化学反应式是:2CuO+C
2Cu+CO2↑.
故答案为:2CuO+C
2Cu+CO2↑;
(3)(1)M是长期露置于空气中的,所以H来源于水,C来源于二氧化碳.由钢铁的生锈可判断氧气也可能参与,故答案为:H2O、CO2;O2;
(3)因为所用蒸馏水都是经煮沸迅速冷却的,A~E的实验来探究生锈的条件,只有A中会生锈,且A与E比较说明O2参加了反应.故选AE.
故答案为:①铝(或Al);
(2)碳还原氧化铜生成铜和二氧化碳,化学反应式是:2CuO+C
| ||
故答案为:2CuO+C
| ||
(3)(1)M是长期露置于空气中的,所以H来源于水,C来源于二氧化碳.由钢铁的生锈可判断氧气也可能参与,故答案为:H2O、CO2;O2;
(3)因为所用蒸馏水都是经煮沸迅速冷却的,A~E的实验来探究生锈的条件,只有A中会生锈,且A与E比较说明O2参加了反应.故选AE.
点评:题考查学生对地壳里各元素的含量知识的理解与掌握,及正确书写化学方程式的能力.以及利用已有知识的基础上来探究金属生锈的原理,可培养学生的探究能力、实验分析能力、综合解决问题的能力.

练习册系列答案
 互动课堂系列答案
互动课堂系列答案 激活思维智能训练课时导学练系列答案
激活思维智能训练课时导学练系列答案
相关题目