��Ŀ����
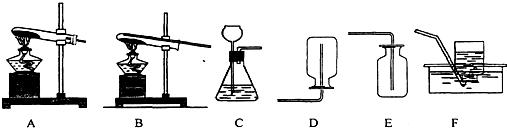 22������ʵ��װ��ͼ���ش��������⣺
22������ʵ��װ��ͼ���ش��������⣺��1��ʵ���������ſ������ռ�������ѡ��
E
װ�ã��ñ�Żش���ͬ��������2��ʵ��������ˮ�����ƹ�������ʯ�Ҽ�����ȡ�������壮������ܶȱȿ���С��
������ˮ��ѡ��
A
װ���Ʊ����飬�ռ�����ʱ��ѡ��
D
��
F
����3����ͼ��ijѧ����Ƶ�һ����ϴ������������;��װ�ã� ����ȥ02�л��е�ˮ������ƿ�п�ʢ
Ũ����
��������������ˮ�����壬������ˮ�������ռ�����ʱ��ƿ����װ��ˮ�������
b
���a����b������ͬ��������ͨ�룮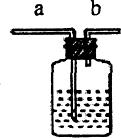
��Ҫ��ˮ��ƿ�������ų�ʹ�ã�ˮӦ��
a
�����ܽ��룮���پٳ���װ�õ�һ����;
ƿ��װ��NaOH��Һ�����ڳ�ȥ�����е�CO2
����������1��ʵ������ȡ���壬����װ�õ�ѡ��Ҫ����Ӧ���״̬�ͷ�Ӧ����������Ӧ���ǹ��壬��Ӧ��Ҫ����ʱ����Aװ�ã�����Ӧ���ǹ����Һ�壬��Ӧ����Ҫ����ʱ����Cװ��
��2���ռ�װ�õ�ѡ��Ҫ��������ܶȺ��ܽ��ԣ�������ȿ������ܶȴ�ʱ���������������ռ�����������ܶȱȿ������ܶ�Сʱ���������������ռ��������岻����ˮʱ������ˮ���ռ���
��3��Ũ���������ˮ�ԣ��ܳ�ȥ�����е�ˮ��������ȥˮ����ʱ��Ҫ�����̳���
��2���ռ�װ�õ�ѡ��Ҫ��������ܶȺ��ܽ��ԣ�������ȿ������ܶȴ�ʱ���������������ռ�����������ܶȱȿ������ܶ�Сʱ���������������ռ��������岻����ˮʱ������ˮ���ռ���
��3��Ũ���������ˮ�ԣ��ܳ�ȥ�����е�ˮ��������ȥˮ����ʱ��Ҫ�����̳���
����⣺��1�������ȿ������ܶȴʴ�Ϊ��E
��2������ˮ�����ƹ�������ʯ�Ҽ�����ȡ�������壮������ܶȱȿ���С����������ˮ����ѡA��D��F
��3��Ũ���������ˮ�ԣ��ʴ�Ϊ��ŨH2SO4��b��a��ƿ��װ��NaOH��Һ�����ڳ�ȥ�����е�CO2���˴𰸲�Ψһ��
��2������ˮ�����ƹ�������ʯ�Ҽ�����ȡ�������壮������ܶȱȿ���С����������ˮ����ѡA��D��F
��3��Ũ���������ˮ�ԣ��ʴ�Ϊ��ŨH2SO4��b��a��ƿ��װ��NaOH��Һ�����ڳ�ȥ�����е�CO2���˴𰸲�Ψһ��
����������ͼ������װ��ʱ���������ȿ������ܶ�С����̽�������

��ϰ��ϵ�д�
�����Ŀ






 ��ijѧ����Ƶ�һ����ϴ������������;��װ�ã�������ˮ�������ռ�����ʱ��ƿ����װ��ˮ�������
��ijѧ����Ƶ�һ����ϴ������������;��װ�ã�������ˮ�������ռ�����ʱ��ƿ����װ��ˮ�������