��Ŀ����
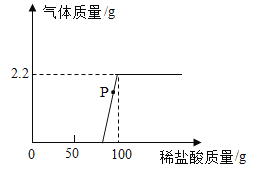
����Ŀ��ʵ������һ����������п�ۺ�̼�۵�̼��Ʒ�ĩ����ѧ��ȤС���ͬѧ�����������Ȥ��Ϊ��֤����ɣ���ȤС���ͬѧ����ͼ��ʾװ�ã����ֳг�װ������ȥ������������ʵ��̽����

��1���������������ƿ�в�����������ϡ���ᣬ��ַ�Ӧ�����ҡ��������ֱ��ռ����������壬���Ҵ����嵼�����ʯ��ˮ��ʯ��ˮ����ǣ����������徭�鴿�����ڿ����а�����ȼ�ա�
��д�������Ҵ���������Ļ�ѧ����ʽ____________���ɴ�֤��������к���̼��ơ�
��д������������������Ļ�ѧ����ʽ_____________���ɴ�֤��������к���п��
��2�����״���Ӧ���ʣ������ˡ����ﲢ������ͭ��ĩ���¼��ȣ��۲쵽��ɫ��ĩ��졣ʵ��֤���û����ȷʵ����_________�����ѧʽ��
���𰸡�![]()
![]() C
C
��������
��1���ٽ��Ҵ����嵼�����ʯ��ˮ��ʯ��ˮ����ǣ�˵�������˶�����̼��˵����̼��ƣ�̼�������ϡ���ᷴӦ�����Ȼ��ơ�������̼��ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ��![]() ��
��
�ڱ��������徭�鴿�����ڿ����а�����ȼ�գ�˵�����ɵ���������������֤��������к�п��п����ϡ���ᷴӦ�����Ȼ�п���������÷�Ӧ�Ļ�ѧ����ʽΪ��![]() ��
��
��2�����״���Ӧ���ʣ������ˡ����ﲢ������ͭ��ĩ���¼��ȣ��۲쵽��ɫ��ĩ��죬˵��������к�̼������ͭ��̼�ڸ��µ������·�Ӧ������ͭ�Ͷ�����̼�����C��