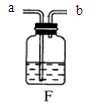��Ŀ����
��5�֣�ijУ��ѧ��ȤС��ѧϰ���������ȡ���ռ������֪ʶ�����ܽᣬ����һ
����룬�����������Ŀ���ݣ�
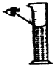
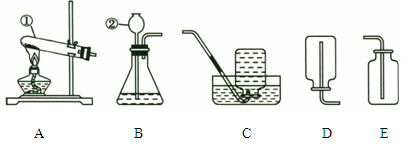
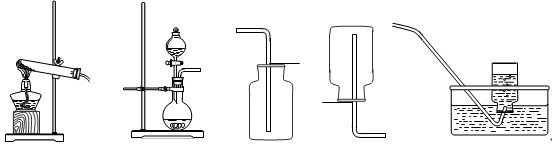
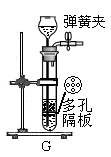
��1��д�������������ƣ�a ��
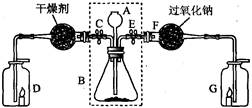
��2������Eװ���ռ�������������Ӧ�� ��ͨ�루�c����d������
��3��װ��B��C����������ȡ������̼���壬װ��C�����װ��B�ڲ�������������ǣ�
������F�ռ�CO2��Ҫ�������ɵ�CO2����������������ˮ���Ϸ�һ��ֲ����Ŀ���� ��
��д��ʵ������ȡCO2�Ļ�ѧ����ʽ�� ��
����룬�����������Ŀ���ݣ�

��1��д�������������ƣ�a ��
��2������Eװ���ռ�������������Ӧ�� ��ͨ�루�c����d������
��3��װ��B��C����������ȡ������̼���壬װ��C�����װ��B�ڲ�������������ǣ�
������F�ռ�CO2��Ҫ�������ɵ�CO2����������������ˮ���Ϸ�һ��ֲ����Ŀ���� ��
��д��ʵ������ȡCO2�Ļ�ѧ����ʽ�� ��
(1)�ƾ��� ��2��C
(3)�ܿ��Ʒ�Ӧ�ķ�����ֹͣ ��ֹ������̼�ܽ���ˮ�в������
(4) CaCO3+2HCl==CaCl2+H2O+CO2��
(3)�ܿ��Ʒ�Ӧ�ķ�����ֹͣ ��ֹ������̼�ܽ���ˮ�в������
(4) CaCO3+2HCl==CaCl2+H2O+CO2��
���������(1)����������ʶ��
(2)װ��E���ڡ�����ƿ���� ����Eװ���ռ���������Ϊ�������ܶȱȿ������ܶȴ�������Ӧ��c ��ͨ��
(3) װ��C��һ���������ϸ��壬������ʵ�ֹ�Һ���룬����װ��C�����װ��B�ڲ�������������ǣ��ܿ��Ʒ�Ӧ�ķ�����ֹͣ����Ҫ������Ӧ���ѻ����������Һ��Ӵ�����Ӧ��������Ҫ��Ӧ���رջ����������������Ų�������ʹװ����ѹǿ�������Һ����룬��Ӧֹͣ������F�ռ�CO2��Ҫ�������ɵ�CO2�������������ڶ�����̼������ˮ������Ҫ��ˮ���Ϸ�һ��ֲ���ͣ�Ŀ���Ƿ�ֹ������̼�ܽ���ˮ�в������
��4��ʵ������ȡCO2�Ļ�ѧ����ʽ�ǣ�CaCO3+2HCl==CaCl2+H2O+CO2��

��ϰ��ϵ�д�
�����Ŀ