��Ŀ����
����Ŀ��ij�о�С��Ϊ̽����Ӱ��������ʴ���������ء���ȡ����ˮ�ͺ�ˮ����ͼװ����ɱ���ʵ�飬�ش��������⡣
ʵ����� | �� | �� | �� | �� |
ʵ���¶�/�� | 30 | 30 | 60 | 60 |
�Լ� | ����ˮ | ��ˮ | ����ˮ | ��ˮ |
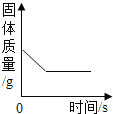
�������� ����ʱ�� | 1�� δ����ʴ | 10���� | 1�� δ����ʴ | 5���� |
��1���������Ҫ�ɷ���______�����ѧʽ����
��2��ʵ��ںܿ͢�̽��______���ض�����Ʒ��ʴ������Ӱ�죻ʵ��______����ʵ����ţ���̽���Լ������ʶ�����Ʒ��ʴ������Ӱ�졣
��3�����ݱ���ʵ������ó��Ľ�����______ ����д��1�㼴�ɣ���
��4��Ӱ������Ʒ��ʴ���������������⣬���������������ʵ�鷽����֤��IJ���______��
���𰸡�Fe2O3 �¶� �ٺ͢�(��ۺ͢�) ������������ͬ�������£��¶�Խ�ߣ�����ʴ������Խ�죬�ο��Լӿ�������ʴ���� ȡ��ͬ�����������Թ��У�����������ˮ�������������Թ���ͨ�벻ͬŨ�ȵ�������Ȼ��۲������������õ�ʱ��
��������
(1)�������Ҫ�ɷ�����������
(2)ʵ��ں͢��е��Լ���Ϊ��ˮ�������¶Ȳ�ͬ������̽���¶ȶ�����Ʒ��ʴ������Ӱ�죻ʵ��ٺ͢����¶���ͬ���ۺ͢����¶���ͬ�������Լ���ͬ������̽���Լ������ʶ�����Ʒ��ʴ������Ӱ�죻
(3)���ݱ���ʵ�������֪��������������ͬ�������£��¶�Խ�ߣ�����ʴ������Խ�죬�ο��Լӿ�������ʴ���ʣ�
(4)Ӱ������Ʒ��ʴ���������������⣬����������Ũ�ȣ���Ƶ�ʵ�鷽���ǣ�ȡ��ͬ�����������Թ��У�����������ˮ�������������Թ���ͨ�벻ͬŨ�ȵ�������Ȼ��۲������������õ�ʱ�䡣

����Ŀ�������������������뿪�����ʡ�ij��ȤС����������о����£�
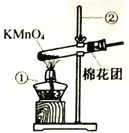
I.�������Ʊ���
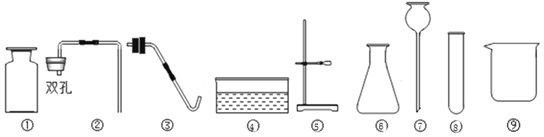
(1)д����ͼ���б�����������ƣ���___________����__________��
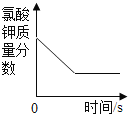
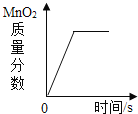
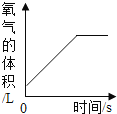
(2)д����KMnO4��ȡO2�Ļ�ѧ��Ӧ����ʽ__________________����װ�������ŵ�������_________________��ͼ��װ��һ�����ԵĴ�����______________��
II.�������ռ���
[ʵ��1]�������ſ������ռ��������������ڼ���ƿ�ڴ�����ľ����ȼʱֹͣ�ռ����ⶨƿ�������ĺ������ظ�ʵ��3�Ρ�
[ʵ��2]�������ſ������ռ��������������ڼ���ƿ�ڴ�����ľ����ȼ�����ռ�40�룬�ⶨƿ�������ĺ������ظ�ʵ��3�Ρ�
[ʵ��3]����ˮ���ռ��������ⶨƿ�������ĺ������ظ�ʵ��3�Ρ�
ʵ������:
ʵ��1 | ʵ��2 | ʵ��3 | |||||||
�������������(%) | 79.7 | 79.6 | 79.9 | 88.4 | 89.0 | 87.9 | 90.0 | 89.8 | 89.3 |
������ƽ���������(%) | 79.7 | 88.4 | 89.7 | ||||||
����
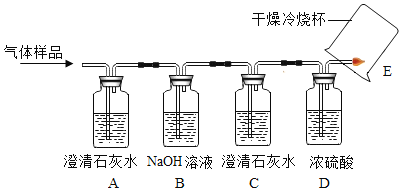
(3)��ʵ��1��2��֪���������ſ������ռ�����ʱ��Ϊ���õ���������������ɲ�ȡ�Ĵ�ʩ��_______________��
(4)�����Dz������أ�ʵ��3��õ���������������ܴﵽ100%����Ҫԭ����_______��
��.��˿��������ȼ��
(5)��˿�ڴ���������ȼ�յĻ�ѧ��Ӧ����ʽΪ________________________��
(6)��˿ȼ��ʱ�������䣬���о����������������������ԭ������ͬʱ������ij�����壬�Ʋ��������_____________(�ѧʽ)����ȼ�պ�ĺ�ɫ�������μ�ϡ���ᣬ���������ݣ���ԭ����________________(�û�ѧ��Ӧ����ʽ��ʾ)��
����Ŀ����һ�����ı���(C3H8O)����������һ����յ���������ȼ����÷�Ӧǰ������ʵ��������±���
���� | ���� | ���� | ˮ | ������̼ | X |
��Ӧǰ����/g | 6.0 | 12.8 | 0 | 0 | 0 |
��Ӧ������/g | 0 | 0 | 7.2 | 8.8 | a |
�����ж���ȷ���ǣ�������
A. X��һ����̼B. Xһ���Ǹ÷�Ӧ�Ĵ���
C. X���ܺ�����Ԫ��D. ����a��ֵΪ2g