��Ŀ����
Ϊ������Դ����״��������ҳ����ʮ����滮��̨��
��1��ҳ��������Ȼ������Ҫ�ɷݶ��Ǽ��飬�����ڿ�������ȫȼ�յĻ�ѧ����ʽΪ_________����Ȼ����ú��_______�ϳ�Ϊ����ʯȼ�ϡ�
��2��ҳ�����������ı��ҹ����ڵ���Դ��֣�Ϊ����������������Ӱ�졣���л���������ȼúû��ֱ�ӹ�ϵ����___________������ĸ����
A������ B������ЧӦ C���������ƻ� D�����������������
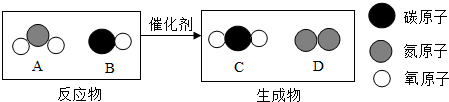
��3������ β���Ǵ�����Ⱦ��Ҫ��Դ֮һ������Ч��ת�������ɽ�����β�����ж����崦��Ϊ�����壬��ͼΪ�÷�Ӧ����ʾ��ͼ�� �ش��������⡣
��1��ҳ��������Ȼ������Ҫ�ɷݶ��Ǽ��飬�����ڿ�������ȫȼ�յĻ�ѧ����ʽΪ_________����Ȼ����ú��_______�ϳ�Ϊ����ʯȼ�ϡ�
��2��ҳ�����������ı��ҹ����ڵ���Դ��֣�Ϊ����������������Ӱ�졣���л���������ȼúû��ֱ�ӹ�ϵ����___________������ĸ����
A������ B������ЧӦ C���������ƻ� D�����������������
��3������ β���Ǵ�����Ⱦ��Ҫ��Դ֮һ������Ч��ת�������ɽ�����β�����ж����崦��Ϊ�����壬��ͼΪ�÷�Ӧ����ʾ��ͼ�� �ش��������⡣
��A������Ԫ��������Ϊ_______________��
��4�������У����ڻ��������___________����ͼ����ĸ����
���ڸ÷�Ӧ�У�����C��D��������Ϊ____________������������������ȱ�ʾ����
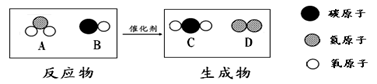
��4�������У����ڻ��������___________����ͼ����ĸ����
���ڸ÷�Ӧ�У�����C��D��������Ϊ____________������������������ȱ�ʾ����
��1��CH4 +2O2 CO2 +2H2O��ʯ��
CO2 +2H2O��ʯ��
��2��C
��3����7:16 ����ABC ����44:7
 CO2 +2H2O��ʯ��
CO2 +2H2O��ʯ����2��C
��3����7:16 ����ABC ����44:7

��ϰ��ϵ�д�
 Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д�
�����Ŀ