��Ŀ����
�������ʶ�������ˮ�ʵľ�����ɱ��������
��1�������ͻ���̼����ˮ�м������������ܽ⣬����һ��ʱ����� ����������ƣ����Ӷ���ȥ����С�������������õij���Һ�м������̿��������
��ȥˮ�е���ɫ����ζ��
��2������(��ѧʽO3)����ǿ�����ԣ���������Ӿ�ء�������ˮ����ˮ��ɱ�����������������������Ԫ����ͬ����ѧ���ʲ�ͬ��ԭ���� ��
��3��Ư�ۣ�������ˮ��ɱ������������Ч�ɷ��Ǵ������[��ѧʽΪCa(ClO)2]��������ƿɷ������·�Ӧ��Ca(ClO)2 + X + H2O��CaCO3��+2HClO����X�Ļ�ѧʽΪ ��
��4��ClO2������ˮ������ClO2��������ȡClO2�ķ�Ӧ���۹�����ͼ��ʾ������![]() ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ�![]() ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ�![]() ��ʾ��ԭ�ӣ���
��ʾ��ԭ�ӣ���
��д����Ӧ�Ļ�ѧ����ʽ ��
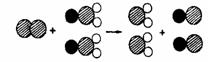
��1������ ������ ��2�����ӹ��ɲ�ͬ ��3��CO2
��4��Cl2 + 2NaClO2 = 2ClO2 + 2NaCl

��ϰ��ϵ�д�
 53���ò�ϵ�д�
53���ò�ϵ�д�
�����Ŀ
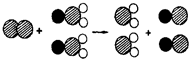 25���������ʶ�������ˮ�ʵľ�����ɱ��������
25���������ʶ�������ˮ�ʵľ�����ɱ�������� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�����д����Ӧ�Ļ�ѧ����ʽ
��ʾ��ԭ�ӣ�����д����Ӧ�Ļ�ѧ����ʽ ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ���
��ʾ��ԭ�ӣ���
 ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ���
��ʾ��ԭ�ӣ���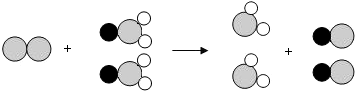 ��д����Ӧ�Ļ�ѧ����ʽ
��д����Ӧ�Ļ�ѧ����ʽ ��ʾ��
��ʾ��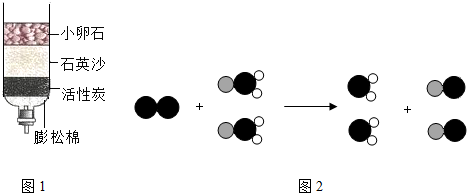
 ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�����д����Ӧ�Ļ�ѧ����ʽ
��ʾ��ԭ�ӣ�����д����Ӧ�Ļ�ѧ����ʽ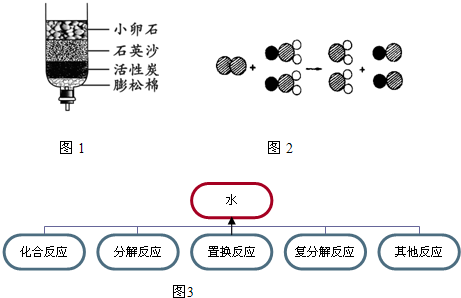
 ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ�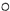 ��ʾ��ԭ�ӣ�����д����Ӧ�Ļ�ѧ����ʽ
��ʾ��ԭ�ӣ�����д����Ӧ�Ļ�ѧ����ʽ