��Ŀ����
���������Ҫ�������Դ�����õĻ�������1���ҹ��Ϻ��̲��ŷḻ�ġ���ȼ������Դ������ȼ�����ijɷ��Ǽ���ˮ����
�������й�CH4?xH2O��˵����ȷ���� ������ĸ����
a����һ�ֻ���� b���ڵ��¸�ѹ�³ʹ�̬
c��̼Ԫ�������������ڼ��� d��C��H��Oԭ�Ӹ�����Ϊ2��6��1
�ڿ�ȼ������ ����ɡ����ߡ����ɡ���������Դ��
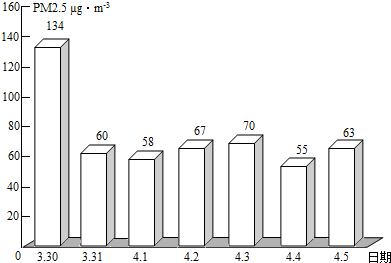
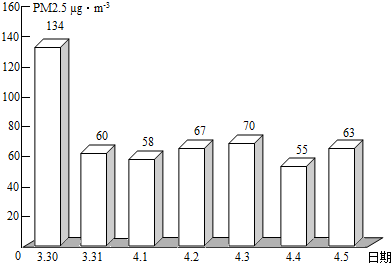
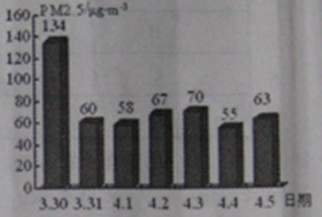
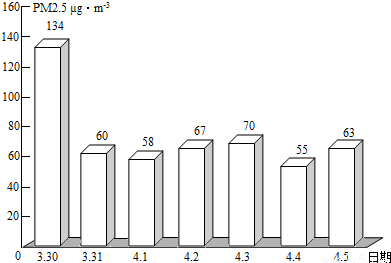
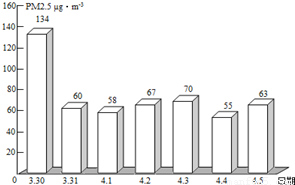
��2��PM2.5��ʾÿ����������ֱ����2.5��m�Ŀ����ﺬ�����ҹ����������ġ������������������涨����ס���������24Сʱ�ڵ�PM2.5ƽ��Ũ����75µg/m3֮��Ϊ��꣮ͼΪ��ͨһ��PM2.5��ʵʱ������ݣ�
�ٴ�ͼ�п�֪������PM2.5���� �죬���д�ʩ�в��ܽ��Ϳ�����PM2.5���� ������ĸ����
a�������� b����ֹ���սո� c��ֲ������
��3������Ҳ�ǻ�������֮һ�������pHֵ ������������������ߡ�=����7��
�γ��������Ҫ���ʣ�����úȼ�ղ����� ���壨�ѧʽ��������β���ŷŲ����ĵ������NOx���ȣ�
��4�������������������������Ϊ���ǹ�ע���ȵ㣮���д�ʩ�����ϻ���������� ��
A������������̫���ܡ�ˮ�ܡ����ܵ���Դ
B�����������ϡ���������ˮ�������������ŷ�
C��Ϊʹũ����߲�������ũ�����ʩ�û��ʺ�ũҩ��
���𰸡���������1������Ŀ����Ϣ��֪������ȼ�����ijɷ��Ǽ���ˮ����ڵ��¸�ѹ�³ʹ�̬�����ڻ������ȼ�����ڲ�����������Դ��
��2����ͼ�п�֪������PM2.5����һ�죬�ܽ��Ϳ�����PM2.5���ǣ���ֹ���սոѡ�ֲ�����ֵȣ�
��3��PHֵС��5.6����ˮ��Ϊ���ꣻ����������Ҫ��Դ��ú��ʯ�͵Ȼ�ʯȼ�ϵ�ȼ�գ������ŷŵķ����ȣ����������꣮
��4����������ָ����������Ϣʱ�����õ�����Ҫ�������٣�������Ⱦ����ŷţ��ر��Ǽ��ٶ�����̼���ŷ�����������̬�����Դӽڵ硢���ܺͻ��յȻ������ı�����������ϸ�ڣ��ݴ˽��з�����ɣ�
����⣺��1������ȼ�����ijɷ��Ǽ���ˮ����ڵ��¸�ѹ�³ʹ�̬�����ڻ������ȼ�����ڲ�����������Դ��
�ʴ�Ϊ����b���ڲ��ɣ�
��2����ͼ�п�֪������PM2.5����һ�죬�ܽ��Ϳ�����PM2.5���ǣ���ֹ���սոѡ�ֲ�����ֵȣ�
�ʴ�Ϊ����1����a��
��3��PHֵС��5.6����ˮ��Ϊ���ꣻ����������Ҫ��Դ��ú��ʯ�͵Ȼ�ʯȼ�ϵ�ȼ�գ������ŷŵķ����ȣ����������ꣻ
�ʴ�Ϊ���٣�����SO2��
��4��A������������̫���ܡ�ˮ�ܡ����ܵ���Դ�����Խ�Լ��Դ�������������ϻ��������⣻
B�����������ϡ���������ˮ�������������ŷţ����Ա���ˮ��Դ�����ϻ������⣻
C��Ϊʹũ����߲�������ũ�����ʩ�û��ʺ�ũҩ������Ⱦ������ˮ��Դ�������ϻ��������⣮
�ʴ�Ϊ��C��
�����������������й���Դ�����⣬��Լ��ʯ��Դ�������Ŀ�������Դ�ǿ�ѧ���������о����¿��⣬�й���Դ����Ҳ�ǽ������п����ȵ�֮һ��ͬѧ��Ҫ������գ�
��2����ͼ�п�֪������PM2.5����һ�죬�ܽ��Ϳ�����PM2.5���ǣ���ֹ���սոѡ�ֲ�����ֵȣ�
��3��PHֵС��5.6����ˮ��Ϊ���ꣻ����������Ҫ��Դ��ú��ʯ�͵Ȼ�ʯȼ�ϵ�ȼ�գ������ŷŵķ����ȣ����������꣮
��4����������ָ����������Ϣʱ�����õ�����Ҫ�������٣�������Ⱦ����ŷţ��ر��Ǽ��ٶ�����̼���ŷ�����������̬�����Դӽڵ硢���ܺͻ��յȻ������ı�����������ϸ�ڣ��ݴ˽��з�����ɣ�
����⣺��1������ȼ�����ijɷ��Ǽ���ˮ����ڵ��¸�ѹ�³ʹ�̬�����ڻ������ȼ�����ڲ�����������Դ��
�ʴ�Ϊ����b���ڲ��ɣ�
��2����ͼ�п�֪������PM2.5����һ�죬�ܽ��Ϳ�����PM2.5���ǣ���ֹ���սոѡ�ֲ�����ֵȣ�
�ʴ�Ϊ����1����a��
��3��PHֵС��5.6����ˮ��Ϊ���ꣻ����������Ҫ��Դ��ú��ʯ�͵Ȼ�ʯȼ�ϵ�ȼ�գ������ŷŵķ����ȣ����������ꣻ
�ʴ�Ϊ���٣�����SO2��
��4��A������������̫���ܡ�ˮ�ܡ����ܵ���Դ�����Խ�Լ��Դ�������������ϻ��������⣻
B�����������ϡ���������ˮ�������������ŷţ����Ա���ˮ��Դ�����ϻ������⣻
C��Ϊʹũ����߲�������ũ�����ʩ�û��ʺ�ũҩ������Ⱦ������ˮ��Դ�������ϻ��������⣮
�ʴ�Ϊ��C��
�����������������й���Դ�����⣬��Լ��ʯ��Դ�������Ŀ�������Դ�ǿ�ѧ���������о����¿��⣬�й���Դ����Ҳ�ǽ������п����ȵ�֮һ��ͬѧ��Ҫ������գ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ