��Ŀ����
����Ŀ��ijʵ��С���о����ᡢ�������ơ����������������ʵĻ�ѧ���ʣ�������ͼ��ʾ��ʵ�飬�μӵ�ҩƷ����������������ͼ������ҩƷ������һ������о���
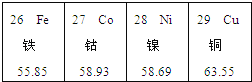
��1��ʵ���ij�Թ���Ϊ��ɫ��Һ�����Թ��з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2��ʵ��� �Թ���Ϊ��ɫ��Һ��������ĸ��
��3��ʵ���ij�Թܵĵײ��а�ɫ���壬���˺�����Һ�еμ�ϡ���ᣬ��ʵ������Ϊ ��
��4��ʵ�����ʱ�����ǽ�ʵ���Һ����ͬһ��Һ���У��۲�����Dz������յķ�Һ���ܳ����ԣ�Ϊ����֤���룬��������ʵ����δ�漰������Ļ�ѧ���ʣ���ѡҩƷ����ʵ�飺ȡ������Һ��Ʒ���Թ��У������м���һ������ ���۲�����֤�����յķ�Һ�����ԣ�ʵ���Ϊ�˱����Һ��ɲ�����������ǶԷ�Һ������ǡ��������
���𰸡���1��Fe2O3+6HCl=2FeCl3+3H2O����2��CF��
��3����������������ݲ�������4������
����������1����������ϡ���ᷴӦ�����Ȼ�����ˮ���Ȼ�������ˮ�ʻ�ɫ���ʻ�ѧ����ʽΪ��Fe2O3 + 6HCl = 2FeCl3 + 3H2O��
��2��������̼���Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼������Ϊ��ɫ�����������������ᷴӦ�����Ȼ��ƺ�ˮ����ҺΪ��ɫ�����CF��
��3���������ƺ�̼���Ʒ�Ӧ����̼��Ƴ������������ƣ����˺����ϡ���ᣬ����ʯ��ˮ�������ʼ�������û����������̼��ʹ�����ʯ��ˮ����ǣ����ڶ�����̼���������в���̼���ת��Ϊ̼����ƣ��ʼ��������۲쵽�����ݲ����������������������ݲ�����
��4�����ý��������ᷴӦ�������壬��ȡ������Һ��Ʒ���Թ��У������м���һ���������ۣ����������ݣ���֤����Һ�����ԣ�������ۣ�

 ��У����ϵ�д�
��У����ϵ�д�