��Ŀ����
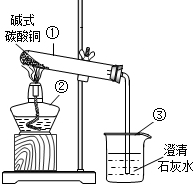
 ��ͼ�Ǽ��ȼ�ʽ̼��ͭ��ʵ��װ��ͼ���ش��������⣺
��ͼ�Ǽ��ȼ�ʽ̼��ͭ��ʵ��װ��ͼ���ش��������⣺��1��д��������ŵ��������ƣ���
�Թ�
�Թ�
��������̨
����̨
�����ձ�
�ձ�
�����ƾ���
�ƾ���
����2�����Ⱥ��Թ۲쵽�Թ���
��
��
ɫ��ĩ�����
��
ɫ���Թܱ��ϳ���ˮ��
ˮ��
��ͬʱ���Կ�������ʯ��ˮ�����
�����
����3���û�ѧ���ţ���ѧʽ����ʾ��ʽ̼��ͭ���ȷ�Ӧ�Ĺ��̣�Cu2��OH��2CO3
| �� |
CuO
CuO
+H2O
H2O
+CO2
CO2
��4��ͼ�����Դ�����
�Թ��ڵ������̫��������������ĵ���
�Թ��ڵ������̫��������������ĵ���
����������1��Ҫ��Ǹ������������ƣ�
��2����ʽ̼��ͭ����ʱ����ɫ��ĩ��ɺ�ɫ���Թܱ�����ˮ����֣�ʯ��ˮ����ǣ�
��3����ʽ̼��ͭ���ȷֽ�����������ͭ��ˮ�Ͷ�����̼��
��4������ʵ�����������ע������������
��2����ʽ̼��ͭ����ʱ����ɫ��ĩ��ɺ�ɫ���Թܱ�����ˮ����֣�ʯ��ˮ����ǣ�
��3����ʽ̼��ͭ���ȷֽ�����������ͭ��ˮ�Ͷ�����̼��
��4������ʵ�����������ע������������
����⣺��1���٢ڢܢݷֱ����Թܡ�����̨���ձ����ƾ��ƣ�
��2�����Ⱥ��Թ۲쵽�Թ�����ɫ��ĩ��ɺ�ɫ���Թܱ��ϳ���ˮ�飬ͬʱ���Կ�������ʯ��ˮ����ǣ�
��3����ʽ̼��ͭ���ȷֽ�Ļ�ѧ����ʽΪ��Cu2��OH��2CO3
CuO+CO2��+H2O��
��Ӧ����һ�֣������������֣����ڷֽⷴӦ������ֽⷴӦ��
��4�����ȼ�ʽ̼��ͭ���ɶ�����̼���壬Ҫ��������ĵ������Թ��ڵĵ��ܲ�Ӧ���̫����
�ʴ�Ϊ����1���Թܣ�����̨���ձ����ƾ��ƣ�
��2���̣��ڣ�ˮ�飻����ǣ�
��3��CuO��CO2��H2O��
��4���Թ��ڵ������̫��������������ĵ�����
��2�����Ⱥ��Թ۲쵽�Թ�����ɫ��ĩ��ɺ�ɫ���Թܱ��ϳ���ˮ�飬ͬʱ���Կ�������ʯ��ˮ����ǣ�
��3����ʽ̼��ͭ���ȷֽ�Ļ�ѧ����ʽΪ��Cu2��OH��2CO3
| �� |
��Ӧ����һ�֣������������֣����ڷֽⷴӦ������ֽⷴӦ��
��4�����ȼ�ʽ̼��ͭ���ɶ�����̼���壬Ҫ��������ĵ������Թ��ڵĵ��ܲ�Ӧ���̫����
�ʴ�Ϊ����1���Թܣ�����̨���ձ����ƾ��ƣ�
��2���̣��ڣ�ˮ�飻����ǣ�
��3��CuO��CO2��H2O��
��4���Թ��ڵ������̫��������������ĵ�����
������������Ҫ����ʵ������ͻ�ѧʽ����д�ȷ����֪ʶ���������֪ʶ������ȷ���

��ϰ��ϵ�д�
�����Ŀ
 ��ͼ�Ǽ��ȼ�ʽ̼��ͭ��ʵ��װ��ͼ���ش��������⣺
��ͼ�Ǽ��ȼ�ʽ̼��ͭ��ʵ��װ��ͼ���ش��������⣺ 30����ͼ�Ǽ��ȼ�ʽ̼��ͭ��ʵ��װ��ͼ���Իش��������⣺
30����ͼ�Ǽ��ȼ�ʽ̼��ͭ��ʵ��װ��ͼ���Իش��������⣺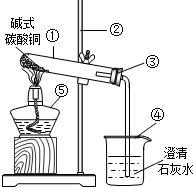 ��ͼ�Ǽ��ȼ�ʽ̼��ͭ��ʵ��װ��ͼ���Իش��������⣺
��ͼ�Ǽ��ȼ�ʽ̼��ͭ��ʵ��װ��ͼ���Իش��������⣺