��Ŀ����
��2005?��ͨ����ͼ��1994���2004��ij���е�һ�����ʱ�̲�Ŀ����ж�������ĺ���������˵��������ǣ� ��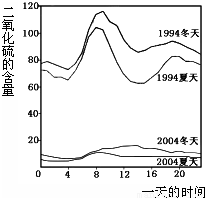
���𰸡�������A���۲�ͼʾ��֪����������еĶ���������������ߣ�
B���۲�1994�궬������������ͼ�жϣ�
C�������еĶ���������Ҫ����ú�Ȼ�ʯȼ�ϵ�ȼ�պ����Ṥ���ŷŵ�β����
D�����ݶ����������Դ�ͷ����Ŀ����Իش�
����⣺A���۲�ͼʾ��֪��������ʾ��������еĶ���������������ߣ���ȷ��
B���۲�ͼʾ��֪��������ʾ��1994��һ���д�Լ8�����Ҷ�������ĺ����ϸߣ���ȷ��
C�����ݿ����еĶ���������Ҫ����ú�Ȼ�ʯȼ�ϵ�ȼ�պ����Ṥ���ŷŵ�β����֪����������ĺ�����10��併�͵�ԭ������Ǽ�ǿ��ȼ�ϵ�����Ϳ����˶���������ŷţ�����ȷ��
D������������Ҫ����ú�Ȼ�ʯȼ�ϵ�ȼ�պ����Ṥ���ŷŵ�β�����ھ��ǽ�ֹʹ�������Dz���ʵ�ʵģ��ʴ���
��ѡD��
������Ҫ���ٴ����ж�������ĺ���������ǿ��ȼ�ϵ�����Ϳ����˶���������ŷ����⣬��Ӧ�������͵Ļ�����Դ���Ӷ������������Ⱦ���⣮
B���۲�1994�궬������������ͼ�жϣ�
C�������еĶ���������Ҫ����ú�Ȼ�ʯȼ�ϵ�ȼ�պ����Ṥ���ŷŵ�β����
D�����ݶ����������Դ�ͷ����Ŀ����Իش�
����⣺A���۲�ͼʾ��֪��������ʾ��������еĶ���������������ߣ���ȷ��
B���۲�ͼʾ��֪��������ʾ��1994��һ���д�Լ8�����Ҷ�������ĺ����ϸߣ���ȷ��
C�����ݿ����еĶ���������Ҫ����ú�Ȼ�ʯȼ�ϵ�ȼ�պ����Ṥ���ŷŵ�β����֪����������ĺ�����10��併�͵�ԭ������Ǽ�ǿ��ȼ�ϵ�����Ϳ����˶���������ŷţ�����ȷ��
D������������Ҫ����ú�Ȼ�ʯȼ�ϵ�ȼ�պ����Ṥ���ŷŵ�β�����ھ��ǽ�ֹʹ�������Dz���ʵ�ʵģ��ʴ���
��ѡD��
������Ҫ���ٴ����ж�������ĺ���������ǿ��ȼ�ϵ�����Ϳ����˶���������ŷ����⣬��Ӧ�������͵Ļ�����Դ���Ӷ������������Ⱦ���⣮

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
��2005?��ͨ���ҹ�����ר�Һ�°�ġ������Ƽ��Ϊ�����Ƽҵ������ͻ���Ĺ��ף�����ʳ�Ρ�ˮ��������������̼Ϊԭ�ϣ����Ƶ�̼�����ƺ��Ȼ�泥���������������±��Ǽ��������ڲ�ͬ�¶�ʱ���ܽ�ȣ�
��1���ӱ������ݿ��Կ������¶ȶԵ��ܽ��Ӱ�첻��
��2��ҪʹNH4Cl������Һ�е�NH4Cl�ᾧ�������ڣ���ϸߡ��ϵ͡����¶��½��бȽϺ��ʣ�
��3���������Ƽ����NaHCO3�ķ���ʽ��NaCl+CO2+NH3+H2O�TNaHCO3+NH4Cl���÷�Ӧ�ܽ��е�ԭ���ǣ�
��1���ӱ������ݿ��Կ������¶ȶԵ��ܽ��Ӱ�첻��
��2��ҪʹNH4Cl������Һ�е�NH4Cl�ᾧ�������ڣ���ϸߡ��ϵ͡����¶��½��бȽϺ��ʣ�
| 0�� | 20�� | 40�� | 60�� | |
| NaHC03 | 6.9 | 9.6 | 2.7 | 6.4 |
| NaCl | 35.7 | 35.8 | 6.6 | 37.3 |
| NH4Cl | 29.4 | 37.2 | 45.8 | 55.2 |

 ������Һ�е�
������Һ�е� �ᾧ��������________(��ϸߡ��ϵ͡�)�¶��½��бȽϺ��ʣ�
�ᾧ��������________(��ϸߡ��ϵ͡�)�¶��½��бȽϺ��ʣ�

 �ķ���ʽ��
�ķ���ʽ�� ���÷�Ӧ�ܽ��е�ԭ����________��
���÷�Ӧ�ܽ��е�ԭ����________��