��Ŀ����
����Ŀ��������,�ҹ���Щ����������Ⱦ������,��Ҫԭ������Ϊ����ȼ�պ������ߵ�ú���������ŷŵ�β������ɵ�.
��������⣩ú���Ƿ�����Ԫ�غ�̼Ԫ��?
���������ϣ�1.����������ʹ���������Һ��ɫ(���Ϻ�ɫ��Ϊ��ɫ)���÷�Ӧ�Ļ�ѧ����ʽΪ: 5SO2+2KMnO4+2x=K2SO4+2MnSO4+2H2SO4����x�Ļ�ѧʽΪ___________,
�ڶ�����̼������������ʹ����ʯ��ˮ�����,��д��������̼�����ʯ��ˮ��Ӧ�Ļ�ѧ����ʽ_______
������������,�ס���ͬѧ������ʵ��̽��
(1)��ͬѧ��������ͼ��ʾA��B����ʵ�����۲쵽��ʵ��������
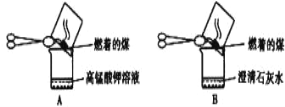
A��_________________,B�г���ʯ��ˮ����ǡ�
�ó�������úȼ�����ɶ�������Ͷ�����̼��֤��ú�к�����Ԫ�غ�̼Ԫ��
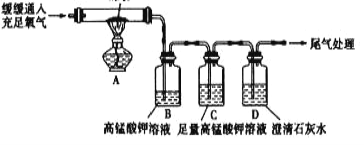
(2)��ͬѧ��Ϊ��ͬѧ�ķ���������,������_________________________������������ͼ��ʾʵ��(�г�װ����ȥ��)
�������뷴˼��
(3)��ͼ��Cװ�õ�������_______________.
(4)��ͬѧ��ʵ����֤��ú�к�����Ԫ�غ�̼Ԫ�ص�������,B��_____C��____D��____.
���𰸡�H2O CO2 +Ca(OH)2 =CaCO3��+ H2O ���������Һ��ɫ ��������Ҳ��ʹ����ʯ��ˮ����ǣ���֤��ú�к���̼Ԫ�� ������������Ƿ�������ų���������ĸ��� ��Һ��ɫ ��Һ����ɫ �����ʯ��ˮ�����
��������
����������
�����ݷ�Ӧ�Ļ�ѧ����ʽ5SO2+2KMnO4+2x=K2SO4+2MnSO4+2H2SO4�������غ㶨�ɣ���Ӧǰ���ԭ���������Ŀ���䣩�жϣ���Ӧ��K2SO4��2MnSO4��2H2SO4�й�����3����ԭ�ӡ�2����ԭ�ӡ�2����ԭ�ӡ�20����ԭ�ӡ�4����ԭ�ӣ���Ӧǰ5SO2��2KMnO4�й�����5����ԭ�ӡ�2����ԭ�ӡ�2����ԭ�ӡ�18����ԭ�ӡ�0����ԭ�ӣ�2X��Ӧ����4����ԭ�ӡ�2����ԭ����һ��X������Ӧ����2����ԭ�ӡ�1����ԭ�ӣ�����X�Ļ�ѧʽ��H2O��
�ڶ�����̼���������Ʒ�Ӧ����̼��ư�ɫ������ˮ����Ӧ�Ļ�ѧ����ʽΪ��CO2 +Ca(OH)2 =CaCO3��+ H2O��
ʵ��̽����
��1������������ʹ���������Һ��ɫ�����Ϻ�ɫ����ɫ��������ʵ��A�е������Ǹ��������Һ��ɫ��
��2�����ݲ������ϡ�������̼������������ʹ����ʯ��ˮ����ǡ���֪����������Ҳ��ʹ����ʯ��ˮ����ǣ���֤��ú�к���̼Ԫ�أ�
�����뷴˼��
��3�����������֪����������Զ�����̼�ļ�����ڸ��ţ�ͼ��Cװ�õ������Ǽ�����������Ƿ�������ų���������ĸ��ţ�
��4����ͬѧ��ʵ����֤��ú�к�����Ԫ�غ�̼Ԫ������ͨ��ʹ���������Һ��ɫ֤���ж��������ٳ�ȥ������������֤������̼���ɣ���B�е���Һ��ɫ��C�е���Һ����ɫ��D�г����ʯ��ˮ����ǡ�