题目内容
(2006?下关区一模)合理利用化学反应可以造福人类.请写出下列反应的化学方程式.
(1)实验室制备氧气
(2)利用氢氧化钠溶液洗涤石油产品中残余硫酸
(3)长期盛放石灰水的瓶子内壁常附有一层白色物质.发生反应的方程式是
(4)用氢氧化钠溶液吸收硫在氧气中燃烧实验放出的二氧化硫气体
(1)实验室制备氧气
2KMnO4
K2MnO4+MnO2+O2↑
| ||
2KMnO4
K2MnO4+MnO2+O2↑
.
| ||
(2)利用氢氧化钠溶液洗涤石油产品中残余硫酸
2NaOH+H2SO4=Na2SO4+2H2O
2NaOH+H2SO4=Na2SO4+2H2O
.(3)长期盛放石灰水的瓶子内壁常附有一层白色物质.发生反应的方程式是
Ca(OH)2+CO2═CaCO3↓+H2O
Ca(OH)2+CO2═CaCO3↓+H2O
.(4)用氢氧化钠溶液吸收硫在氧气中燃烧实验放出的二氧化硫气体
2NaOH+S02=Na2S03+H20
2NaOH+S02=Na2S03+H20
.分析:根据题意,找出反应物、反应条件、生成物,根据化学方程式的书写步骤正确书写化学方程式.
解答:解:(1)实验室用高锰酸钾加热分解制备氧气,高锰酸钾在加热的条件下生成锰酸钾、二氧化锰和氧气,故此化学方程式为:2KMnO4
K2MnO4+MnO2+O2↑;
(2)氢氧化钠和硫酸反应生成硫酸钠和水,反应的化学方程式为:2NaOH+H2SO4=Na2SO4+2H2O;
(3)石灰水的溶质是氢氧化钙,碱可以和酸性氧化物反应生成盐和水.氢氧化钙和二氧化碳生成碳酸钙沉淀和水,反应的化学方程式:Ca(OH)2+CO2═CaCO3↓+H2O.
(4)二氧化硫与氢氧化钠反应生成亚硫酸钠和水,反应的化学方程式为:2NaOH+S02=Na2S03+H20;
故答案为:(1)2KMnO4
K2MnO4+MnO2+O2↑;(2)2NaOH+H2SO4=Na2SO4+2H2O;(3)Ca(OH)2+CO2═CaCO3↓+H2O;(4)2NaOH+S02=Na2S03+H20;
| ||
(2)氢氧化钠和硫酸反应生成硫酸钠和水,反应的化学方程式为:2NaOH+H2SO4=Na2SO4+2H2O;
(3)石灰水的溶质是氢氧化钙,碱可以和酸性氧化物反应生成盐和水.氢氧化钙和二氧化碳生成碳酸钙沉淀和水,反应的化学方程式:Ca(OH)2+CO2═CaCO3↓+H2O.
(4)二氧化硫与氢氧化钠反应生成亚硫酸钠和水,反应的化学方程式为:2NaOH+S02=Na2S03+H20;
故答案为:(1)2KMnO4
| ||
点评:在解此类方程式的书写题时,首先确定反应原理,然后再依据原理找出反应物、生成物和反应条件,根据方程式的书写规则书写方程式.

练习册系列答案
 口算题卡加应用题集训系列答案
口算题卡加应用题集训系列答案 综合自测系列答案
综合自测系列答案
相关题目
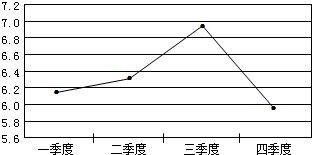 (2006?下关区一模)在一年中不同时期,对秦淮河某段检测河水的平均pH值变化,根据记录得到如图所示的折线图.那么,这一年内此段河水酸性最弱的季度是( )
(2006?下关区一模)在一年中不同时期,对秦淮河某段检测河水的平均pH值变化,根据记录得到如图所示的折线图.那么,这一年内此段河水酸性最弱的季度是( )