��Ŀ����
 ��2013?ƽ��ɽһģ��������;�㷺����ṹ�����ʵ��ǻ�ѧ����Ҫ�о����ݣ�
��2013?ƽ��ɽһģ��������;�㷺����ṹ�����ʵ��ǻ�ѧ����Ҫ�о����ݣ���1����ͼ1������ԭ�ӽṹʾ��ͼ������˵������ȷ����
A
A
��A����ԭ���ڷ�Ӧ���õ���
B���ڻ���������ͨ����+3��
C�����ǵؿ��к������Ľ���Ԫ��
D���������������������������õĵ�����
��2��ij��ѧС����һ����NaNO3��Cu��NO3��2�����Һ������ͼ2ʵ�飬������ҺA����B�ijɷֽ����˷�����ʵ��̽����
[�������]��ҺA�е����ʿ�������Щ��
[��������]��ֻ��Zn��NO3��2 ��Zn ��NO3��2��Cu��NO3��2 ��Zn ��NO3��2��NaNO3
[��������]�����IJ�����
��
��
�����ţ�����������Zn����NaNO3�뷴Ӧ����������һ������NaNO3
Zn����NaNO3�뷴Ӧ����������һ������NaNO3
��[ʵ��̽��]ͨ������ʵ���ȷ������B�ijɷ֣��뽫����д������
| ʵ�鲽�� | �� �� | �йط�Ӧ�Ļ�ѧ����ʽ |
| ȡ����B�������μ� ϡ���ᣨ��ϡ����ȣ� ϡ���ᣨ��ϡ����ȣ� |
�����ݲ��� | Zn+2HCl=ZnCl2+H2�� ����Zn+H2SO4=ZnSO4+H2���� Zn+2HCl=ZnCl2+H2�� ����Zn+H2SO4=ZnSO4+H2���� |
��ȡCO
��ȡCO
���������ɵĻ�ѧ����ʽΪFe2O3+3CO
2Fe+3CO2
| ||
Fe2O3+3CO
2Fe+3CO2
��
| ||
��4����ͭ��Cu-Zn�Ͻ�20g��100gϡ�������ձ���ǡ����ȫ��Ӧ����Ӧ�����ձ���ʣ�����������Ϊ119.6g������ϡ���������ʵ�������������������ȷ��0.1%��
��������1��A��������ԭ�������ĵ��������ص������
B���������Ļ��ϼ۷�����
C���������ڵؿ��еĺ���������
D���������ĵ����Է�����
��2��[��������]���ݽ������˳�����Ӧ�÷������룻
[ʵ��̽��]���ݽ������˳�����Ӧ�����ʵ��̽��B�ijɷ֣�
��3������̼�����ʷ���������¯�н�̿�����ã�д�������ķ�Ӧ����ʽ��
��4�����������غ㶨������������������ٸ���п������ķ�Ӧ���������������������Һ�����ʵ��������Ϳ����������ϡ���������ʵ�����������
B���������Ļ��ϼ۷�����
C���������ڵؿ��еĺ���������
D���������ĵ����Է�����
��2��[��������]���ݽ������˳�����Ӧ�÷������룻
[ʵ��̽��]���ݽ������˳�����Ӧ�����ʵ��̽��B�ijɷ֣�
��3������̼�����ʷ���������¯�н�̿�����ã�д�������ķ�Ӧ����ʽ��
��4�����������غ㶨������������������ٸ���п������ķ�Ӧ���������������������Һ�����ʵ��������Ϳ����������ϡ���������ʵ�����������
����⣺��1��A����ԭ�ӵ�������������3������4�����ڻ�ѧ��Ӧ����ʧȥ���ӣ�˵������
B���ڻ���������ͨ����+3�ۣ�˵����ȷ��
C�����ǵؿ��к������Ľ���Ԫ�أ�˵����ȷ��
D���������������������������õĵ����ԣ�˵����ȷ��
��2��[��������]�ɽ������˳�����Ӧ�ÿ�֪��Zn����NaNO3�뷴Ӧ����������һ������NaNO3�����ԣ������IJ����Ǣۣ�
[ʵ��̽��]�ɽ������˳�����Ӧ�ÿ�֪��B��һ����ͭ������п����ķ�Ӧ��ȷ���Ƿ���п�����ʵ�����£�
��3��������¯�н�̿��������ͨ��ȼ���ṩ��������ȡCO���������ɵĻ�ѧ����ʽΪ��Fe2O3+3CO
2Fe+3CO2��
��4��������������20g+100g-119.6g=0.4g
��ϡ���������ʵ���������Ϊx%��
Zn+H2SO4=ZnSO4+H2��
98 2
100g��x% 0.4g
=
��ã�x%=19.6%
�ʴ�Ϊ����1��A����2���ۣ�Zn����NaNO3�뷴Ӧ����������һ������NaNO3
��3����ȡCO��Fe2O3+3CO
2Fe+3CO2����4��ϡ���������ʵ���������Ϊ19.6%��
B���ڻ���������ͨ����+3�ۣ�˵����ȷ��
C�����ǵؿ��к������Ľ���Ԫ�أ�˵����ȷ��
D���������������������������õĵ����ԣ�˵����ȷ��
��2��[��������]�ɽ������˳�����Ӧ�ÿ�֪��Zn����NaNO3�뷴Ӧ����������һ������NaNO3�����ԣ������IJ����Ǣۣ�
[ʵ��̽��]�ɽ������˳�����Ӧ�ÿ�֪��B��һ����ͭ������п����ķ�Ӧ��ȷ���Ƿ���п�����ʵ�����£�
| ʵ�鲽�� | ���� | �йط�Ӧ�Ļ�ѧ����ʽ |
| ϡ���ᣨ��ϡ����ȣ� | Zn+2HCl=ZnCl2+H2�� ����Zn+H2SO4=ZnSO4+H2���� |
| ||
��4��������������20g+100g-119.6g=0.4g
��ϡ���������ʵ���������Ϊx%��
Zn+H2SO4=ZnSO4+H2��
98 2
100g��x% 0.4g
| 98 |
| 2 |
| 100g��x% |
| 0.4g |
�ʴ�Ϊ����1��A����2���ۣ�Zn����NaNO3�뷴Ӧ����������һ������NaNO3
| ʵ�鲽�� | ���� | �йط�Ӧ�Ļ�ѧ����ʽ |
| ϡ���ᣨ��ϡ����ȣ� | Zn+2HCl=ZnCl2+H2�� ����Zn+H2SO4=ZnSO4+H2���� |
| ||
���������⿼���˽������˳���Ӧ�ã���ɴ��⣬�������ݽ������˳����������У�

��ϰ��ϵ�д�
�����Ŀ
 ��2013?ƽ��ɽһģ����ͼ�Ǽס������ֹ�����ܽ�����ߣ�����˵����ȷ���ǣ�������
��2013?ƽ��ɽһģ����ͼ�Ǽס������ֹ�����ܽ�����ߣ�����˵����ȷ���ǣ�������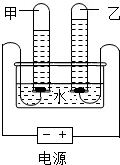 ��2013?ƽ��ɽһģ������ͼװ�õ��ˮ��һ��ʱ����������ͼ��ʾ���Ը�ʵ�������������ǣ�������
��2013?ƽ��ɽһģ������ͼװ�õ��ˮ��һ��ʱ����������ͼ��ʾ���Ը�ʵ�������������ǣ�������